Steroid Detector
by David Bradley
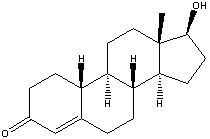 While athletes warm up for that great event, those less than scrupulous participants who try to improve their performance with a dose of the steroid nandrolone better watch out. British scientists have come up with an inexpensive and easy technique that can detect minute quantities of nandrolone in a sample.
While athletes warm up for that great event, those less than scrupulous participants who try to improve their performance with a dose of the steroid nandrolone better watch out. British scientists have come up with an inexpensive and easy technique that can detect minute quantities of nandrolone in a sample.
The new nandrolone detector has been built by Carl Percival and his team of physicists and chemists at Nottingham Trent University. It can detect the tiniest quantities of nandrolone in a liquid sample down to tens of parts per billion.
Anabolic steroids, look and work like the male hormone testosterone. Sports authorities ban them because they give the user more than a sporting chance by artificially helping them boost muscle strength and cut down recovery times after strenuous training. Sprinter Ben Johnson was famously disgraced for taking anabolic steroids, while many other athletes, from runner Linford Christie to tennis player Petr Korda have tested positive for
nandrolone.
It is not only the integrity of sporting events that are at risk when athletes use steroids, they also put their own long-term health at risk. Steroid abuse has been linked to the development of inappropriate secondary sex characteristics, such as breast growth in men, who may also suffer impotence because of abuse. Women abusers sometimes develop masculine facial hair and suffer baldness. Moreover, steroids have been linked with liver damage, heart disorders and even cancer.
According to Percival, a quick and effective way of screening many samples for anabolic steroids, such as nandrolone, is needed to help stamp out steroid abuse in sport. He and his team reckon that, when fully developed, their device could do the trick. The secret to the device's sensitivity is a tiny piece of polymer into which is imprinted the three-dimensional shape of the nandrolone molecule. When the polymer is exposed to a solution containing nandrolone, the nandrolone molecules slot neatly into the imprinted holes and stick to the polymer.
But, the molecules are obviously far too small for the eye to see, so the researchers use a clever piece of microscale engineering to find out whether the holes are filled or not.
The second component of the detector, exploits the same principle that keeps a quartz watch running on time. It consists of a tiny vibrating quartz crystal on to which the imprinted polymer is fixed during the fabrication process. Detection then involves measuring the frequency of vibration. As nandrolone molecules stick to the imprinted polymer, the mass on the vibrating crystal increases and slows the vibration. The decrease is only small, just a couple of parts per million difference in frequency, but that is enough to be picked up by a sensitive electronic detector.
The researchers have used standard solutions of nandrolone to calibrate this change in the vibrational frequency of the quartz crystal with the concentration of the steroid in the solution. They have compared the test results with a blank detector that had a polymer coating without the nandrolone imprint. There was no frequency change for the blank device in nandrolone solution.
Percival and his colleagues publish more details of their nandrolone detector in a forthcoming issue of The Analyst. "We have not got to the stage of testing on 'real' (i.e. urine) samples or developing the hardware for making it a small hand-held device. We have, however, provided a proof of principle," explains team member Mike Newton.
The device will eventually be used in screening, which tells you that something is probably there above a certain quantity but not precisely how much, measuring actual concentration requires a much more detailed and expensive analysis. "Screening should provide an instant test to highlight which sample to send to a lab for further analysis and which should be regarded as clean," adds Newton.
A patent is pending on the device and the team is now seeking a commercial
partner.
Back to the home page
The CaMel and the Jumbo
by David Bradley
 Transparency in sharing and transferring information across the Internet is a must. Today, a spectroscopists's data-stream flows as readily as the outpourings of the Human Genome Project. But, there is one group of scientists that could have become marginalized if it was not for the pioneering work of a handful of their number - the chemists.
Transparency in sharing and transferring information across the Internet is a must. Today, a spectroscopists's data-stream flows as readily as the outpourings of the Human Genome Project. But, there is one group of scientists that could have become marginalized if it was not for the pioneering work of a handful of their number - the chemists.
The problem with chemistry is how to represent what chemists are talking about. In a lecture, the chemistry Professor can easily scratch and scribble a molecular structure, adding notes on the chalkboard and hopefully the students will understand. But, the sharing of chemical information around the globe is not so trivial. Graphics programs lack chemical savvy, so once drawn the molecule has none of the atom connectivity of the molecule.
Chemists do have their own drawing packages that retain inherent knowledge about a molecule drawn using them, such as ChemDraw and ChemSketch and files can usually be interconverted via standards such as the mol file format. But, chemistry is richer than its structural representations.
There are countless nuances that might be associated with a structure, it's molecular weight, the distribution of isotopic ratios of its carbon atoms, its infrared, ultraviolet, nuclear magnetic resonance spectra, its X-ray crystallographic data, the list goes on. How can this additional information be brought to hand transparently?
Thankfully, an answer is forthcoming. CML - chemical mark-up language. The eXtensible Mark-up Language provides a universal format for structured documents and data on the Web and so offers a way for scientists and others to carry a wide range of information types across the net in a transparent way. All that is needed is an XML browser. "XML is a framework from which problem-specific formats derive," explains CML programmer and organic chemist Beda Kosata of the Prague Institute of Chemical Technology.
But, there remains a barrier. How can the seeming simplicity of XML carry all those atomic coordinates, spectra and connectivity information in a transparent manner? The clue lies in the semantics, it is not called eXtensible for nothing, and in recent years a pioneering group of chemists has developed a chemical language system under the XML format to allow chemical information to be transported easily and retrieved and displayed on a user's screen with just those few clicks as other scientists have achieved with their knowledge base.
CML represents a sea change in the management of molecular information, it has been described as "HTML for molecules" but it is so much more, having the scope to span disciplines from the smallest inorganic molecules, carbon monoxide, water and ozone, to vast macromolecular structures, such as polymers, proteins and DNA, and it can handle quantum chemistry. Its nickname as "HTML for molecules" hints at its potential to bring together disparate chemical information sources - those connectivity, spectra, and X-ray data - into a coherent and structured document format without loss of chemical nous. In doing so chemists are granted a far greater freedom of expression.
 Chemical Internet pioneers Peter Murray-Rust, Henry Rzepa and Christopher Leach introduced the concept of a Chemical Mark-up Language at the American Chemical Society meeting in August 1995. They realised that molecular modelling programs, like MOPAC, generate interesting chemical information only in human readable form. The output is not "self defining", and worse, explains Rzepa, was prone to change between versions. "By making a special version of MOPAC 'CML aware' (done by my student Chris Leach) and hence capable of both reading and writing the early CML we showed how information could be made to 'round trip' not only in MOPAC but through any chemical information system that could "add value" to chemistry."
Chemical Internet pioneers Peter Murray-Rust, Henry Rzepa and Christopher Leach introduced the concept of a Chemical Mark-up Language at the American Chemical Society meeting in August 1995. They realised that molecular modelling programs, like MOPAC, generate interesting chemical information only in human readable form. The output is not "self defining", and worse, explains Rzepa, was prone to change between versions. "By making a special version of MOPAC 'CML aware' (done by my student Chris Leach) and hence capable of both reading and writing the early CML we showed how information could be made to 'round trip' not only in MOPAC but through any chemical information system that could "add value" to chemistry."
Mark-up languages are nothing without their translator, and Nottingham University virtual chemist Peter Murray-Rust now at the Unilever-Cambridge University molecular informatics centre soon revealed a CML browser written in Tcl/Wish. Gradually, the language's formalisms evolved and with the emergence of the Java platform-independent programming system in 1996, Murray-Rust had brought a new species to light - JUMBO - a CML browser. JUMBO is, naturally, an example of
CML-aware software.
Importantly, the parallel evolution of XML means that XML now supports "documents" and "data" in seamlessly. So, CML can handle many aspects of chemical informatics, data-handling and publication.
Indeed, Rzepa, Murray-Rust and Michael Wright seminally illustrated the publication possibilitities last year in a paper in the New Journal of Chemistry (2001, 618-634). "Here, the molecules and information about them are precisely specified within the body of the article text," explains Rzepa, "if you feed this article to any CML-aware program (such as JUMBO) it should recognise the molecules, and display them appropriately." Rzepa compares this with the Adobe PDF system commonly used by online publishers, which requires a human reader to extract the chemical information from a picture of a molecule. This is, unlike machine-readable chemical information, slow, error prone, non-reusable and susceptible to inducing boredom! "We have also shown how an XML/CML article could be automatically transformed to e.g. Acrobat form for printing, or to another XML language SVG for high quality viewing. It is enabling these 'added value' transformations that XML offers for the first time, and which we believe is why the use of XML and CML so profoundly anticipates a radical revolution in the primary, secondary and tertiary publishing processes."
The CML website provides examples of its handling capabilities including - datafiles, such as the International Union of Crystallography's CIF and the Protein Data Bank PDB formats. It can handle compound data cards, such as those being produced by the SELFML project to associate molecules and mixtures with their physicochemical properties and Materials Safety Data Sheets (MSDS). The system also allows for the lossless interconversion of various older formats, such as the Mol file, Sybil MOL2, JME, XYZ, SMILES, PDB and CIF. And, of course, it can access the log files of those quantum mechanical programs and display sensible graphics and information from them.
 An exciting recent development in the CML world is the creation of a sub-site on the Open Source development site SourceForge.net. SourceForge.net provides a market for free software as well as services for developers including project hosting, version control, bug and issue tracking, project management, backups and archives, and communication and collaboration resources. Such freedom is a key issue according to Jiri Jirat based in Prague and working on the ZVON.org project for Systinet Corporation: "Using CML you become application and vendor independent, you are not bound to a proprietary format, anybody can write their own application based on CML and you can use this format without any legal or copyright problems for data interchange and storage."
An exciting recent development in the CML world is the creation of a sub-site on the Open Source development site SourceForge.net. SourceForge.net provides a market for free software as well as services for developers including project hosting, version control, bug and issue tracking, project management, backups and archives, and communication and collaboration resources. Such freedom is a key issue according to Jiri Jirat based in Prague and working on the ZVON.org project for Systinet Corporation: "Using CML you become application and vendor independent, you are not bound to a proprietary format, anybody can write their own application based on CML and you can use this format without any legal or copyright problems for data interchange and storage."
At present, the CML project hosted by SourceForge.net, is just one of more than 37,000 projected hosted, although unfortunately it has to be categorised under bioinformatics as there is no chemistry or molecular science grouping. The CML project has three administrators Murray-Rust, and Imperial College's Henry Rzepa and Michael Wright. These three are also themselves developers, Rzepa and Murray-Rust having devised the pioneering chemical MIME type for the Internet. Among the other developers are Daniel Zaharevitz, Jirat and colleague Miloslav Nic formerly of the Prague Institute of Chemical Technology. Rzepa explains that the CML project on SourceForge.net will carry all the machinery needed for users to make use of CML. This will include XML libraries and definitions, stylesheets and Java-based tools.
Among the earliest applications CML-aware applications was Peter Ertl's JME chemical editor while the Sourceforge projects, JChemPaint editor and JMol also have CML support and more recently Kosata's BKChem, which bring us full circle. The various packages allow one to create bond-by-bond drawing of molecules, generate structures from templates for common carbon rings, add arrows, apply rich text, and align, scale and rotate molecules. Platform independence is vital, "XML [and by definition] CML itself is designed to be platform independent and multilingual, thus enabling the development of fully internationalized data exchange format for scientists from all over the world," explains
Jirit.
CML software is not all being developed in academia though. Chemaxon's Marvin is a Java-based chemistry package that comes with an editor, conversion utilities for CML and although professionally developed is still free. MarvinSketch/Swing, for instance, is an applet for
 editing and visualizing molecules on a web page while its companion MarvinView/Swing can be used for viewing said molecules.
editing and visualizing molecules on a web page while its companion MarvinView/Swing can be used for viewing said molecules.
Murray-Rust and his colleagues are collaborating with Dan Zaharevitz (at US National Cancer Institute) to create 250, 000 molecules in CML. This will form the core of an open molecular resource, which will collect molecular data both experimental and theoretical. "Increasingly chemists (and non-chemists) will be able to ask 'what does this molecule do? and Why is it important?' and since all the information will be in XML it will be relatively straightforward to answer," Murray-Rust told us. "It is then a short step to 'where can I find a molecule with certain properties?' - a chemical-specific web-search engine," he adds.
CML and its potential continue to evolve rapidly. CML can now cater for numeric and string data in scalar, array, matrix or tabular form. Glossaries can carry run-time code and so allow validation or transformation of chemical data, on the fly. Molecular information within the chemical MIME framework is amenable to CML, so that crystallography, symmetry, chirality, atom and bond types can be recognised. Most of all, though each and every atom and bond represented can carry any number of additional attributes from conformational analysis and molecular dynamics to NMR and X-ray information.
Governments are mandating the use of XML in formal documents so it is likely to become standard practice. XML can assist in regulatory and legal processes, where authenticity and validity is essential. "We recognised this early on," points out Rzepa, "and showed how having a well-defined structure to a document could allow components to have digital signatures attached, signed by the person or authority that was responsible for creating or authorising the information." Adding "trust" to a document can not only increase its value, but potentially allow either humans or automatic software agents (robots) to make decisions based on "chains of trust".
"There is steadily growing interest in CML and there is no alternative, " says Murray-Rust. Today, not only molecular structures but chemical reactions and protein sequences can be handled and the likes of combinatorial chemistry are being subsumed. The bottom line is that having started with a few scratchings on the cave, sorry lecture room, chalkboard, chemists are now in the process of evolving the Internet to allow them to share and discuss the very fundamentals of their science.
Back to the sciencebase home page
A wild recipe for hot soil
by David Bradley
The French have always had a penchant for fungi, but one day you may be more likely to find a cep cleaning up after a terrorist bomb or a nuclear accident than being served up in a wild mushroom
soufflé.
Edible mushrooms might not be the most obvious choice for cleaning up after a nuclear accident or the explosion of a so-called "dirty" bomb, a conventional explosive carrying radioactive material. But, French scientists reckon that a wild mushroom might soak up radioactive caesium-137 ions just as easily as it can olive oil. Caesium-137 was released in vast quantities by the explosion at the Chernobyl nuclear power station eleven years ago and could be a major contaminant from a terrorist dirty bomb.
Removing metal and radioactive contaminants from exposed land is a crucial task. Aside from the immediate threat to the environment and the health of those living on or near such land, toxic metal ions can be carried into the food chain by vegetation growing on the land. One clean-up solution, known as bioremediation, involves deliberately planting plant species that might absorb the metals from the soil and then harvesting plants for safe disposal.
 When it comes to the alkali metal caesium, however, there are no plant species that thrive on soil contaminated with it. So, if not a plant, why not a fungus?
When it comes to the alkali metal caesium, however, there are no plant species that thrive on soil contaminated with it. So, if not a plant, why not a fungus?
Anne-Marie Albrecht-Gary and her colleagues at the Louis Pasteur University and the University of Strasbourg think they have found the solution in the unlikely form of the tasty bay boletus,
Xerocomus badius. "Fungi often exhibit a remarkable ability to accumulate a large variety of elements, ranging from the heaviest of the transition metals such as lead or mercury, to the alkali metals, including radioisotopes like caesium-137," explains Albrecht-Gary in a recent issue of
Chemical Communications. But, she adds, little is known about how these fungi take up such metal ions.
She and her colleagues have studied the chemistry of the two pigments that give the inside of the bay boletus cap its bright yellow colour - norbadione A and badione A. These chemicals can act like molecular crab claws grabbing hold of metal ions in a pincer movement known as chelation. The yellow colour of the pigments provided the team with the means to test how well each latches on to metals, such as ceasium. They exploited the pigments' strong absorbtion of ultraviolet wavelengths to record a spectrum of the free pigment molecules and their spectra in the presence of caesium ions are markedly different. UV spectroscopy coupled with chemical analysis revealed that norbadione A in particular can bind to radioactive caesium very strongly. In fact, so strong is the norbadione claw that it can bind two caesium ions, whereas its weaker sibling badione A only has the strength to grip one at a time.
Albrecht-Gary and her colleagues believe that norbadione gets its strength from a so-called allosteric effect. When one caesium ion enters the claw, the molecule's chemistry changes slightly so that a second gripping position opens up to accept another caesium, working like a double claw. Badione A, on the other hand has only one possible grip.
The researchers believe that norbadione A makes the bay boletus so good at sequestering radioactive caesium ions from the soil in which it grows that it should be the bioremedial agent of choice for removing this hazardous metal from contaminated land. "Obviously, fungi can be very efficient at accumulating toxic metals and radioelements and constitute an excellent and elegant tool for soil bioremediation," says Albrecht-Gary, "However, the limitation on this very potent application is controlled growing of the mushrooms."
While norbadione A will almost certainly have a role in bioremediation, the team has dashed hopes for its medical use in removing toxic metals, such as radioactive caesium-137 cadmium and nickel, from the body. Its grip on other alkali metals, such as the essential minerals sodium and potassium is just too strong.
Of, course one problem remains: what to do with the radioactive fungi…one can hardly cook them in an
omelette.
Back to the home page
Read more
scientific discoveries news,
medical
news headlines, and
chemistry articles
Back to the sciencebase
homepage
Please vote on this page and leave your thoughts here.
|  While athletes warm up for that great event, those less than scrupulous participants who try to improve their performance with a dose of the steroid nandrolone better watch out. British scientists have come up with an inexpensive and easy technique that can detect minute quantities of nandrolone in a sample.
While athletes warm up for that great event, those less than scrupulous participants who try to improve their performance with a dose of the steroid nandrolone better watch out. British scientists have come up with an inexpensive and easy technique that can detect minute quantities of nandrolone in a sample. Transparency in sharing and transferring information across the Internet is a must. Today, a spectroscopists's data-stream flows as readily as the outpourings of the Human Genome Project. But, there is one group of scientists that could have become marginalized if it was not for the pioneering work of a handful of their number - the chemists.
Transparency in sharing and transferring information across the Internet is a must. Today, a spectroscopists's data-stream flows as readily as the outpourings of the Human Genome Project. But, there is one group of scientists that could have become marginalized if it was not for the pioneering work of a handful of their number - the chemists. Chemical Internet pioneers Peter Murray-Rust, Henry Rzepa and Christopher Leach introduced the concept of a Chemical Mark-up Language at the American Chemical Society meeting in August 1995. They realised that molecular modelling programs, like MOPAC, generate interesting chemical information only in human readable form. The output is not "self defining", and worse, explains Rzepa, was prone to change between versions. "By making a special version of MOPAC 'CML aware' (done by my student Chris Leach) and hence capable of both reading and writing the early CML we showed how information could be made to 'round trip' not only in MOPAC but through any chemical information system that could "add value" to chemistry."
Chemical Internet pioneers Peter Murray-Rust, Henry Rzepa and Christopher Leach introduced the concept of a Chemical Mark-up Language at the American Chemical Society meeting in August 1995. They realised that molecular modelling programs, like MOPAC, generate interesting chemical information only in human readable form. The output is not "self defining", and worse, explains Rzepa, was prone to change between versions. "By making a special version of MOPAC 'CML aware' (done by my student Chris Leach) and hence capable of both reading and writing the early CML we showed how information could be made to 'round trip' not only in MOPAC but through any chemical information system that could "add value" to chemistry." An exciting recent development in the CML world is the creation of a sub-site on the Open Source development site SourceForge.net. SourceForge.net provides a market for free software as well as services for developers including project hosting, version control, bug and issue tracking, project management, backups and archives, and communication and collaboration resources. Such freedom is a key issue according to Jiri Jirat based in Prague and working on the ZVON.org project for Systinet Corporation: "Using CML you become application and vendor independent, you are not bound to a proprietary format, anybody can write their own application based on CML and you can use this format without any legal or copyright problems for data interchange and storage."
An exciting recent development in the CML world is the creation of a sub-site on the Open Source development site SourceForge.net. SourceForge.net provides a market for free software as well as services for developers including project hosting, version control, bug and issue tracking, project management, backups and archives, and communication and collaboration resources. Such freedom is a key issue according to Jiri Jirat based in Prague and working on the ZVON.org project for Systinet Corporation: "Using CML you become application and vendor independent, you are not bound to a proprietary format, anybody can write their own application based on CML and you can use this format without any legal or copyright problems for data interchange and storage." editing and visualizing molecules on a web page while its companion MarvinView/Swing can be used for viewing said molecules.
editing and visualizing molecules on a web page while its companion MarvinView/Swing can be used for viewing said molecules. When it comes to the alkali metal caesium, however, there are no plant species that thrive on soil contaminated with it. So, if not a plant, why not a fungus?
When it comes to the alkali metal caesium, however, there are no plant species that thrive on soil contaminated with it. So, if not a plant, why not a fungus?