Advanced photons in science
David Bradley is currently working with Argonne National Laboratory
on a series of articles for the annual report of the ANL's Advanced
Photon Source. For December's Elemental Discoveries, I will be offering
a preview of the advanced science featured in the report.

 Elemental Discoveries
shows how X-rays can reveal some of the
inner secrets of the world around us as seen under the illumination of ANL's Advanced Photon Source:
Elemental Discoveries
shows how X-rays can reveal some of the
inner secrets of the world around us as seen under the illumination of ANL's Advanced Photon Source:
Are films ferroelectric?
Discipline for gold nanocrystals
Photosynthetic systems
Dissecting the atom
Catalytic clues
SAXS and the water channel
Digging in the dirt
Folding Protein Sensors
X-ray movies
Discipline for gold nanocrystals
by David Bradley
Gold nanocrystals are not the most well-behaved of materials. Admittedly,
simply mixing the right ingredients will induce them to self-assemble, but
the microscopic and mesoscopic organization of the nanocrystal superlattices
is not predictable.
Researchers would like to be able to control the formation of nanocrystal
superlattices for a whole range of applications from novel types of
catalysts for speeding up useful chemical reactions to building tiny
components for applications in optoelectronics and nanotechnology. However,
they face a serious problem in trying to tame the structure of the
nanocrystal superlattices as they form. They simply cannot predict the
effect on structure of the numerous different forces between the clusters of
gold atoms and the chemical reagents they use to make the nanocrystals.
 Suresh Narayanan
and Jin Wang of the Advanced Photon Source and Xiao-Min Lin of Argonne's
Materials Science Division and Chemistry Division hope to remedy this
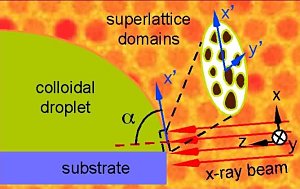
situation. They are using time-resolved small-angle x-ray scattering
measurements on beamline XOR-1BM to take a closer look at the physical
processes taking place as gold nanocrystal superlattices form. They hope
that their insights into these complex processes will allow researchers to
better control them with a view to making gold nanocrystal superlattices of
precise design.
Suresh Narayanan
and Jin Wang of the Advanced Photon Source and Xiao-Min Lin of Argonne's
Materials Science Division and Chemistry Division hope to remedy this
situation. They are using time-resolved small-angle x-ray scattering
measurements on beamline XOR-1BM to take a closer look at the physical
processes taking place as gold nanocrystal superlattices form. They hope
that their insights into these complex processes will allow researchers to
better control them with a view to making gold nanocrystal superlattices of
precise design.
The researchers point out that, in conventional wisdom, gold nanocrystals
can condensed on to a substrate surface from evaporating suspension of gold
nanoparticles and a reactive organic molecule containing sulfur, a
dodecanethiol ligand in organic solvent. As the liquid suspension dries, the
nanoparticles coated with the ligands may self-assemble to produce so-called
superlattices as the liqands act as a kind of spacer. Other researchers have
found that using this approach to nanocrystal construction leads to a wide
range of superlattice structures from ordered two-dimensional and
three-dimensional patterns to fractal-like aggregates and even structures
full of tunnels and channels.
One of the
problems to making desirable two-dimensional nanocrystaluperlattices is a
misunderstanding regarding the mechanism of their formation. Previous
speculations had pointed to the idea that the self-assembly of nanocrystals
occurs at the interface between the liquid and the substrate surface. At
this interface the nanocrystal particles can move freely at the substrate
surface and as the liquid evaporates and de-wets the surface, the
superlattice structures are left behind.
Lin and his colleagues have used transmission electron microscopy to show
that there is too much order for the de-wet idea to hold true. To prove the
point, the team turned to the non-intrusive analytical technique of
small-angle x-ray scattering (SAXS). Using this technique, the researchers
can watch the formation of nanocrystal superlattices as the nanoparticle
suspension droplets evaporates. Their results show that the self-assembly
process does not occur at the interface between the liquid and the substrate
but at the interface of the liquid surface and the surrounding air. They
further demonstrated this to be the case by speeding up the rate of
evaporation and showed that the same starting materials could produce either
two-dimensional or three-dimensional lattices depending on how quickly the
liquid evaporated. The faster rate of evaporation produces 2D structures
whereas a slower rate of evaporation allows time for 3D structures to form.
The self-assembly of 2D nanocrystals superlattices at the liquid-air
interface opens up the possibility of annealling out the defects for
allowing ordered 2D superlattices in mesoscopic scale to form, which has not
been proven possible in the past.
Source: Suresh Narayanan, 1 Jin Wang, 1 and Xiao-Min Lin2 "Dynamical
Self-Assembly of Nanocrystal Superlattices during Colloidal Droplet
Evaporation by in situ Small Angle X-Ray Scattering," Phys. Rev. Lett. 93,
135503 (2004).
Back to the December elemental discoveries index page

 Elemental Discoveries
shows how X-rays can reveal some of the
inner secrets of the world around us as seen under the illumination of ANL's Advanced Photon Source:
Elemental Discoveries
shows how X-rays can reveal some of the
inner secrets of the world around us as seen under the illumination of ANL's Advanced Photon Source: Suresh Narayanan
and Jin Wang of the Advanced Photon Source and Xiao-Min Lin of Argonne's
Materials Science Division and Chemistry Division hope to remedy this
situation. They are using time-resolved small-angle x-ray scattering
measurements on beamline XOR-1BM to take a closer look at the physical
processes taking place as gold nanocrystal superlattices form. They hope
that their insights into these complex processes will allow researchers to
better control them with a view to making gold nanocrystal superlattices of
precise design.
Suresh Narayanan
and Jin Wang of the Advanced Photon Source and Xiao-Min Lin of Argonne's
Materials Science Division and Chemistry Division hope to remedy this
situation. They are using time-resolved small-angle x-ray scattering
measurements on beamline XOR-1BM to take a closer look at the physical
processes taking place as gold nanocrystal superlattices form. They hope
that their insights into these complex processes will allow researchers to
better control them with a view to making gold nanocrystal superlattices of
precise design.