Diamonds are crystalline chunks of carbon, the same stuff as in the 'lead' of a pencil, but they are rare and when cut and polished produce a sparkling and eternal gift for Christmas, or so the adverts say. The adverts have got it wrong, of course, diamonds are anything but eternal - heat them up to a few hundred degrees in air and they will burst into flames. The fact that diamond can react, at least under extreme conditions, does hint that with the right chemistry it might take part in more gentle and potentially useful reactions in the laboratory too.
 Researchers have been making synthetic diamonds for many years for use in grinding and glass cutting. But, they also have some high-tech applications in the microelectronics industry as the cooling heat sinks for high-speed computer chips. The diamond draws heat from even the fastest silicon chip, preventing overheating and helping supercomputers carry out cool calculations. The industry is also looking at building transistors and diodes from diamond that could work at extremely high temperatures or in radioactive environments for military and space applications, where conventional semiconductors might simply burn out. 'This is why the US Department of Defence has funded a lot of diamond research,' explains Robert Hamers of the University of Wisconsin whose team is at the forefront of modifying the surface of diamonds.
Researchers have been making synthetic diamonds for many years for use in grinding and glass cutting. But, they also have some high-tech applications in the microelectronics industry as the cooling heat sinks for high-speed computer chips. The diamond draws heat from even the fastest silicon chip, preventing overheating and helping supercomputers carry out cool calculations. The industry is also looking at building transistors and diodes from diamond that could work at extremely high temperatures or in radioactive environments for military and space applications, where conventional semiconductors might simply burn out. 'This is why the US Department of Defence has funded a lot of diamond research,' explains Robert Hamers of the University of Wisconsin whose team is at the forefront of modifying the surface of diamonds.'Silicon is widely used in microelectronics because it is cheap, but for biochemical sensors the fact that diamond is more chemically robust will probably make it preferable to silicon, says Hamers. Attaching biological molecules and knowing where they are on the gene chip would be easier than with silicon too. A diamond 'gene chips' for biotechnology could detect, sample and sequence huge amounts of DNA. But, for these applications the chemists need to be able to change the surface features more than a scrub and polish will allow.
While diamond is essentially a block of pure carbon the surface carbon atoms are 'tied off' with a hydrogen atom at the very point the crystal is formed. A layer of hydrogen though is not much use in terms of creating a useful device such as a transistor or a chemical sensor. So, chemists are looking at how they might change a diamond's coat. 'Hydrogen-coated diamond is very unreactive and therefore difficult to modify,' explains Douglas Doren, of the University of Delaware, 'but bare diamond surfaces are very reactive.'
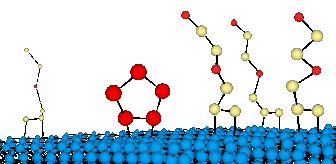
Hamers and colleagues Jennifer Hovis and Sarah Coulter are now working with colleagues at GE Corp in Schenectady, New York, and at the Naval Research Laboratory, in Washington DC have found they can strip a diamond of its hydrogen coat by blasting it at 1600 degrees in a vacuum. When they then pump cyclopentene - molecules of five carbon atoms in a ring - into the vacuum, they attach themselves to the diamond's surface.
The result is millions upon millions of cyclopentene rings linked up with the unstoppered ends of the carbon atoms on the diamond's surface like so many tiny fins jutting out across the surface. Stanford University's George Wang and Stacey Bent also working with the NRL and GE Corp have found they could similarly add another molecule, 1,3-butadiene, to the surface of diamond to make what look like six-carbon 'fins' on the diamond's surface.
Both reactions mean it should be possible to attach other chemical groups, such as DNA or polymer chains, to a diamond. 'We have been working on attaching amines (NH2 groups) and then using these to attach DNA,' explains Hamers. 'The basic idea is to attach single-stranded DNA to the surface, and then to try to detect when this DNA interacts with its complementary strand in solution making the familiar double helix. 'By doing this on diamond, it should be possible to make a direct electronic detector for DNA - kind of like the "tri-corder" in Star Trek,' he muses.
To try and explain what happens during these diamond reactions, Doren and Danesha Fitzgerald, have used computational chemistry to model the way the molecules interact with the diamond surface. They have shown that the addition reactions work because pairs of carbon atoms on the naked surface of the diamond act as if they are themselves individual molecules. Doren adds that there have been several proposals for what to add to the diamond's surface including adding fluorine-containing water-proof and lubricating molecules to the surface of diamonds, giving them what amounts to a Teflon coat. Such materials could be used to build the components of new micromachines, such as cogs, pistons and pumps, for building near-microscopic robots.
However, Fitzgerald and Doren's calculations also revealed that there might be lots of side-products in these reactions. This fact is something the experimentalists could not spot with the analytical techniques used. Nevertheless, the next step is to cover a diamond layer with a molecule, such as cyclopentene, and then find ways to attach other molecules to these appendages. While the chemists might worry about how easy that will be, one wouldn't care to guess what Ms Monroe would have thought of them messing with her best friend.
J. Am. Chem. Soc.