The visible way
By: David Bradley
 The efficient conversion
of sunlight to chemical energy has been until now the preserve of
photosynthesising plants. However, a method for splitting water with
visible light rather than ultraviolet radiation devised by Japanese
researchers could be the key to unlocking artificial photosynthesis.
The efficient conversion
of sunlight to chemical energy has been until now the preserve of
photosynthesising plants. However, a method for splitting water with
visible light rather than ultraviolet radiation devised by Japanese
researchers could be the key to unlocking artificial photosynthesis.
Using the energy of sunlight to release chemical energy in the form
of hydrogen for running fuel cells would provide a cheap and renewable
source of energy for vehicles and both domestic and industrial electricity
production.
Hironori Arakawa of the National Institute of Advanced Industrial Science
and Technology in Tsukuba, Japan, and colleagues there and at the Science
University of Tokyo in Chiba have developed a new mixed catalyst that will extract energy from light of
less than 400 nanometres and allow water to be split. Previously
experimental methods for splitting water with electromagnetic radiation
had relied on the higher energy of ultraviolet.
The team had already demonstrated water splitting with
ultraviolet radiation using a unique synergy effect between two different
titania photocatalysts suspended in aqueous sodium iodide solution. In
that system, hydrogen gas was evolved on a platinum titanate (the mineral
anatase) catalyst while the iodide was oxidised to iodate. Oxygen was
formed on titania (the mineral rutile) as the iodate shuttles back to
iodide.
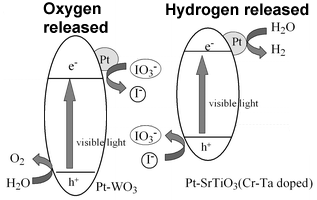 Now, the team has achieved
the same stoichiometric splitting of water into H2 and O2 under visible
light rather than ultraviolet radiation to release hydrogen gas, which
bodes well for the exploitation of sunlight. Non-stoichiometric splitting
would be necessity involve an additional reagent to balance the equation.
Now, the team has achieved
the same stoichiometric splitting of water into H2 and O2 under visible
light rather than ultraviolet radiation to release hydrogen gas, which
bodes well for the exploitation of sunlight. Non-stoichiometric splitting
would be necessity involve an additional reagent to balance the equation.
In the visible version of Hironori's process, the mixed catalyst is
based on platinum tungstate and platinum strontium titanate (doped with
Cr-Ta). The same iodine-based mediator again acts as a shuttle for the
electrons involved in the water splitting.
The team are yet to perfect the catalytic mix to make it produce
the best water splitting efficiency under visible light. "Unfortunately
the quantum efficiency is quite low," concedes Hironori, "we
need to improve the value to 10% or so." He reckons photocatalytic
water splitting technology will mature over the next twenty to thirty
years.
Chem.
Commun.; DOI:
10.1039/b107673f
Back to the main page
Stellar system stifles landfill stench
Anyone living near a landfill will be familiar with the awful smell of
decomposing waste. But, those nasty niffs and disgusting gases could soon
be history, thanks to EPSRC-supported research that is blasting apart the
chemicals responsible using a plasma (see Sparks Fly
below).
The miasma of noxious vapours rising from landfill sites the world over is
responsible for some of the world's worst smells. From the rotten egg
scent of sulphur compounds to the pungent pong of phosphorus-loaded
vegetable matter. There are countless odorous volatiles produced by
millions of tonnes of domestic waste that cloud the opinions of those who
live and work nearby. The problem is a long-term one, 'The smells are not
just produced whilst the landfill site is in operation but continue long
after it is filled and closed, often for decades,' explains Christopher
Whitehead of The University of Manchester.
Whitehead who is supported by a �proof-of-concept� EPSRC grant
- under its Waste and Pollution Management programme - thinks he has come
up with the answer and Accentus plc (part of AEA Technology) is helping to
commercialise the development.
It takes only a tiny quantity of some of the smelliest gases - just
a few molecules among billions to pique the olfactory membranes of the
human nose. Indeed, the nose is far more sensitive to certain molecules
than the best laboratory instrument. But, landfill odour is not just about
offending our delicate noses. Many of the volatiles are linked with
respiratory and environmental problems. If there were a simple way to get
rid of the ethyl butanoate, the halogenated compounds and the malodorous
sulphides, a breath of fresh air could sweep through the waste disposal
industry.
Whitehead is an expert in handling so-called low-temperature gas plasmas.
Plasmas are sometimes described as the fourth state of matter, after
solids, liquids and gases. They are like a gas but instead of being
composed of neutral atoms or molecules, these are ionised so that all
their negatively charged electrons are separate from the positive atomic
nuclei. These electrons are accelerated to high energy in an electric
field and when they collide with the atoms and molecules in the gas, they
create excited states and free radicals that provide a reactive soup that
can destroy the pollutants in the gas.
Plasma is a highly reactive state that can tear molecules apart and
it is this property that could be the end of smelly landfill gases. Pipe
the odourous molecules from a landfill through a plasma and they will be
converted into virtually harmless and odourless compounds, such as carbon
dioxide and water before they are released to the atmosphere. The sulphur,
admittedly, would be released as sulphur dioxide, toxic but significantly
less odorous, but the small quantities present what Whitehead says is 'a
negligible risk'.
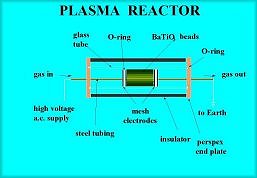 The Whitehead team has been investigating just how feasible it
would be to use a plasma generated by a powerful electric discharge. The
system would work at room temperature producing high-energy electrons,
which decompose the gas into reactive radicals. There would be no costly
to run heating element and because it operates at atmospheric pressure no
reinforced valves and chambers would be needed. The plasma would simply be
produced by a high frequency and high voltage electric discharge like the
ones used to generate ozone for purifying drinking water.
The Whitehead team has been investigating just how feasible it
would be to use a plasma generated by a powerful electric discharge. The
system would work at room temperature producing high-energy electrons,
which decompose the gas into reactive radicals. There would be no costly
to run heating element and because it operates at atmospheric pressure no
reinforced valves and chambers would be needed. The plasma would simply be
produced by a high frequency and high voltage electric discharge like the
ones used to generate ozone for purifying drinking water.
Whitehead's colleague analytical scientist Imtiaz Ahmad is
measuring how well their plasma reactor can destroy odorous molecules. She
uses online FTIR (Fourier-transform infrared spectroscopy) to measure the
'before and after' of mock landfill gas containing a single
odour-producing compound. FTIR provides a characteristic fingerprint of
molecules present. Gas chromatography-mass spectrometry then allows her to
detect the products formed by the plasma. The tests are assessing how well
five common odorous gases are removed from the mock landfill gas.
Among the smelly compounds are halocarbons. 'Halogenated molecules
are common components of many household products,' explains Whitehead,
'and will be found in domestic waste.' He points out that such materials
'may be potential greenhouse gases and represent a threat to the
stratospheric ozone layer.'
 Whitehead emphasises that they will also be looking at the
complicated reaction chemistry of the destruction of the smelly molecules.
This will tell them whether other volatile compounds in real landfill gas
will cause problems with by-products of the plasma process.
Whitehead emphasises that they will also be looking at the
complicated reaction chemistry of the destruction of the smelly molecules.
This will tell them whether other volatile compounds in real landfill gas
will cause problems with by-products of the plasma process.
'We presently have an EPSRC funded (Chemical Engineering) project
to look at the incorporation of real-time computer control into plasma
processing of waste gas streams,' Whitehead told Newsline. The team is
also focusing on using plasma technology for the remediation of waste gas
streams, diesel exhausts and air quality enhancement. 'We will be
particularly involved in understanding and modelling the chemistry
involved in plasma processing as an aid to developing new processes,' he
adds.
With European Union pressure on to cut down on landfill usage
significantly in coming years, a fundamental state of matter found among
the stars could offer a down to earth solution to the problem.
Sparks fly
Plasmas are often associated with more dramatic situations
than landfills. A comet's tail, the solar wind, an astronomical nebula,
lightning, the aurora, even the core of the sun is in a plasma state. In
fact, plasmas are the most common form of matter, making up more than 99%
of the visible universe.
To make a plasma, one either has to heat a low-pressure gas very
strongly - to more than 50 000 Celsius, some eight times hotter than the
surface of the sun. At this temperature, the energy of the gas molecules
or atoms is high enough that the electrons are released from the tight
grip of the nucleus. But, heat isn't the only way to form a plasma, one
can also use an electrical discharge - the gas in a fluorescent lamp forms
a plasma when a high voltage is applied to it. In a non-thermal plasma,
the electrons are at very high temperatures - 10,000 Celsius or more - the
gas atoms and molecules in the gas remain at room temperature.
This article originally appeared in EPSRC Newsline
Back to the main page
A closed outlook on life
In the growing field of research into closed
ecosystems, scientists hope to improve their understanding of what
sustains life. This understanding will help in building life-support
systems for our future journeys into space, and for solving environmental
problems on Earth such as recycling wastewater, purifying air, and growing
food crops in extreme conditions.
 According to Mark Nelson of the Institute of Ecotechnics, London, UK, closed ecological systems research grew from both the mesocosm work studying miniature ecosystems in the laboratory of researchers like University of Florida's Howard Odum and the closed experimental ecospheres (1-10 litre laboratory flasks) created in the 1960s by Clair Folsome at the University of Hawaii. The early Russian and American space programs too made simple bioregenerative closed systems that used algae to recycle air and water and could sustain human life for short periods. The field entered the popular imagination when the Biosphere 2 system, which included many natural ecosystems as well as the agricultural and human living quarters was built in Arizona.
According to Mark Nelson of the Institute of Ecotechnics, London, UK, closed ecological systems research grew from both the mesocosm work studying miniature ecosystems in the laboratory of researchers like University of Florida's Howard Odum and the closed experimental ecospheres (1-10 litre laboratory flasks) created in the 1960s by Clair Folsome at the University of Hawaii. The early Russian and American space programs too made simple bioregenerative closed systems that used algae to recycle air and water and could sustain human life for short periods. The field entered the popular imagination when the Biosphere 2 system, which included many natural ecosystems as well as the agricultural and human living quarters was built in Arizona.
The meeting featured presentations from researchers in Germany, Russia, Japan and the US. Volker Bluem at the University of Bremen and Klaus Slenzka and colleagues at OHB-System GmbH, on the Technology Park at the University of Bremen for instance have developed aquatic organism life-support systems, which have undergone spaceflight experiments. Other papers included bioregenerative recycling of wastewater using highly biodiverse constructed wetlands, a spin-off technology from the Biosphere 2 project authored by Nelson, Odum and colleagues. The Russians continue their innovative work in the field, both in Moscow and at the Institute of Biophysics, Krasnoyarsk, Siberia, and several of their papers described computer modelling and investigations of simple microbial and plant systems.
The Japanese team working on CEEF (Closed Ecological Experiment Facilities) also presented their first experimental data which includes understanding how higher crop plants and domestic animals might respond to environmental changes. Keiji Nitta and colleagues believe CEEF could help in predicting the effect of small temperature rises, perhaps due to global warming, on rice growth. Nitta has also investigated the potential impact on the carbon cycle of small amounts radioactive carbon dioxide that will likely be released into the air by the Rokkasho-Mura nuclear fuel reprocessing plant, which is currently under construction and could still be modified.
The Russian team of Alexander Tikhomirov and Sofiya Ushakova at the Institute of Biophysics (Russian Academy of Science, Siberian Branch) meanwhile have examined how photosynthesis can be affected by different conditions using a bioregenerative life-support system. They have discovered that wheat plants kept in 0 Celsius air can tolerate prolonged periods of darkness much better.
Life faces many problems, understanding how we can sustain it at the extremes - whether under threat of global warming, the harsh Siberian winter, or the depths of space could be one of the greatest scientific challenges of this century.
Papers from the F4.4 Life Science session on Closed Ecological Systems:
Earth and Space Applications are published in [[LINK]]; a major session at the 33rd COSPAR general assembly in Warsaw, Poland.
 The efficient conversion
of sunlight to chemical energy has been until now the preserve of
photosynthesising plants. However, a method for splitting water with
visible light rather than ultraviolet radiation devised by Japanese
researchers could be the key to unlocking artificial photosynthesis.
The efficient conversion
of sunlight to chemical energy has been until now the preserve of
photosynthesising plants. However, a method for splitting water with
visible light rather than ultraviolet radiation devised by Japanese
researchers could be the key to unlocking artificial photosynthesis. Now, the team has achieved
the same stoichiometric splitting of water into H2 and O2 under visible
light rather than ultraviolet radiation to release hydrogen gas, which
bodes well for the exploitation of sunlight. Non-stoichiometric splitting
would be necessity involve an additional reagent to balance the equation.
Now, the team has achieved
the same stoichiometric splitting of water into H2 and O2 under visible
light rather than ultraviolet radiation to release hydrogen gas, which
bodes well for the exploitation of sunlight. Non-stoichiometric splitting
would be necessity involve an additional reagent to balance the equation. The Whitehead team has been investigating just how feasible it
would be to use a plasma generated by a powerful electric discharge. The
system would work at room temperature producing high-energy electrons,
which decompose the gas into reactive radicals. There would be no costly
to run heating element and because it operates at atmospheric pressure no
reinforced valves and chambers would be needed. The plasma would simply be
produced by a high frequency and high voltage electric discharge like the
ones used to generate ozone for purifying drinking water.
The Whitehead team has been investigating just how feasible it
would be to use a plasma generated by a powerful electric discharge. The
system would work at room temperature producing high-energy electrons,
which decompose the gas into reactive radicals. There would be no costly
to run heating element and because it operates at atmospheric pressure no
reinforced valves and chambers would be needed. The plasma would simply be
produced by a high frequency and high voltage electric discharge like the
ones used to generate ozone for purifying drinking water. Whitehead emphasises that they will also be looking at the
complicated reaction chemistry of the destruction of the smelly molecules.
This will tell them whether other volatile compounds in real landfill gas
will cause problems with by-products of the plasma process.
Whitehead emphasises that they will also be looking at the
complicated reaction chemistry of the destruction of the smelly molecules.
This will tell them whether other volatile compounds in real landfill gas
will cause problems with by-products of the plasma process. According to Mark Nelson of the Institute of Ecotechnics, London, UK, closed ecological systems research grew from both the mesocosm work studying miniature ecosystems in the laboratory of researchers like University of Florida's Howard Odum and the closed experimental ecospheres (1-10 litre laboratory flasks) created in the 1960s by Clair Folsome at the University of Hawaii. The early Russian and American space programs too made simple bioregenerative closed systems that used algae to recycle air and water and could sustain human life for short periods. The field entered the popular imagination when the Biosphere 2 system, which included many natural ecosystems as well as the agricultural and human living quarters was built in Arizona.
According to Mark Nelson of the Institute of Ecotechnics, London, UK, closed ecological systems research grew from both the mesocosm work studying miniature ecosystems in the laboratory of researchers like University of Florida's Howard Odum and the closed experimental ecospheres (1-10 litre laboratory flasks) created in the 1960s by Clair Folsome at the University of Hawaii. The early Russian and American space programs too made simple bioregenerative closed systems that used algae to recycle air and water and could sustain human life for short periods. The field entered the popular imagination when the Biosphere 2 system, which included many natural ecosystems as well as the agricultural and human living quarters was built in Arizona.