Some four years ago (Education in Chemistry, Distillates, November 1993), I reported that UK chemists had built a logic gate from a single molecule. Such a device could switch on the development of molecular computers with a million times more power than a silicon chip. We still seem to be quite some way from a real chemical computer but the same team has recently taken an important step forward in the development of their molecular AND gate they have made it fully digital.
An AND gate is a crucial component of computer calculations. It acts as the carry digit during binary addition. To work, however, an AND gate needs two inputs acting independently to produce a strong output signal. It has to be digital so when it is in its 'on' or 'off' state these will represent a '1' and '0' in binary code.
Prasanna 'AP' de Silva and his team at Queen's University Belfast have now tailored their original AND device so that it produces a strong visible glow representing the binary '1' only when two inputs - sodium and potassium ions - are present. 'We have got strong digital behaviour with our AND gate, which is vital for molecular arithmetic - a major goal for the near future,' says de Silva.
de Silva's team built their logical molecule from a crown ether - a ring of alternating oxygen and carbon atoms - the sodium detector - and a fluorescent anthracene unit that can bind a hydrogen ion acting as the second input. The presence of the sodium in the crown ether boosts the glow so that the output is effectively only 'on' when both ions are present in the molecule.
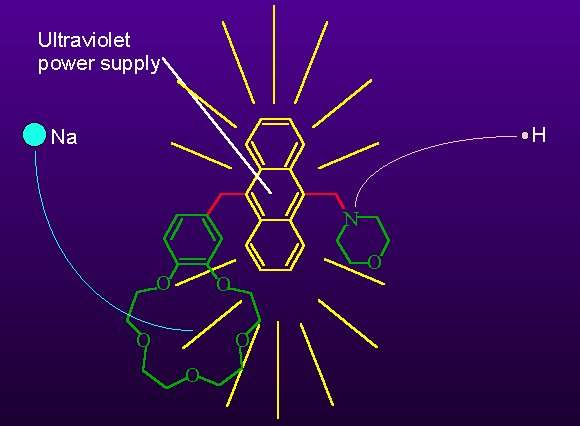
Their previous attempt on the AND gate illustrated the principles explains AP but was not entirely successful because only a weak output signal was produced. 'The present version is the first digital molecular AND gate which is ready for the next big step towards molecular computation,' he enthuses, 'hopefully we will use this type of molecule in arithmetic systems before too long.'
According to supramolecular chemist Marcos Gomez a member of Fraser Stoddart's group at UCLA, 'de Silva has managed to obtain responses to an external source from very simple organic molecules thanks to a very careful chemical design. Not only this, but these molecules can be tuned into giving a YES or NO output depending on two different and independent inputs which allows for dual control of the molecular switching properties. This property makes them prime candidates for the main components of a binary device.'
AP's team is now working on methods of integrating the logic gates without having to link them physically so avoiding any wiring problems!
US chemists have made what seems to be the most twisted hydrocarbon molecule ever. The compound is a polycyclic aromatic hydrocarbon (PAH) - five benzene rings fused edge to edge with an additional one at the edge of one end and with phenyl groups attached along the periphery. The molecule has a twist of 105 degrees, which considering PAHs are normally thought of as flat is rather surprising and could, say the researchers, help in the design of novel molecular architectures.
DNA, several supramolecular compounds and many proteins come with a twist. 'But,' says Robert Pascal of Princeton University in New Jersey, 'when one describes something as twisted, it usually implies that there is an untwisted or flat geometry, which may be more usual or normal. Thus, a twisted ribbon is interesting because ribbons are usually flat. It is in this sense that the twisted PAHs are interesting, since PAHs are usually flat.'
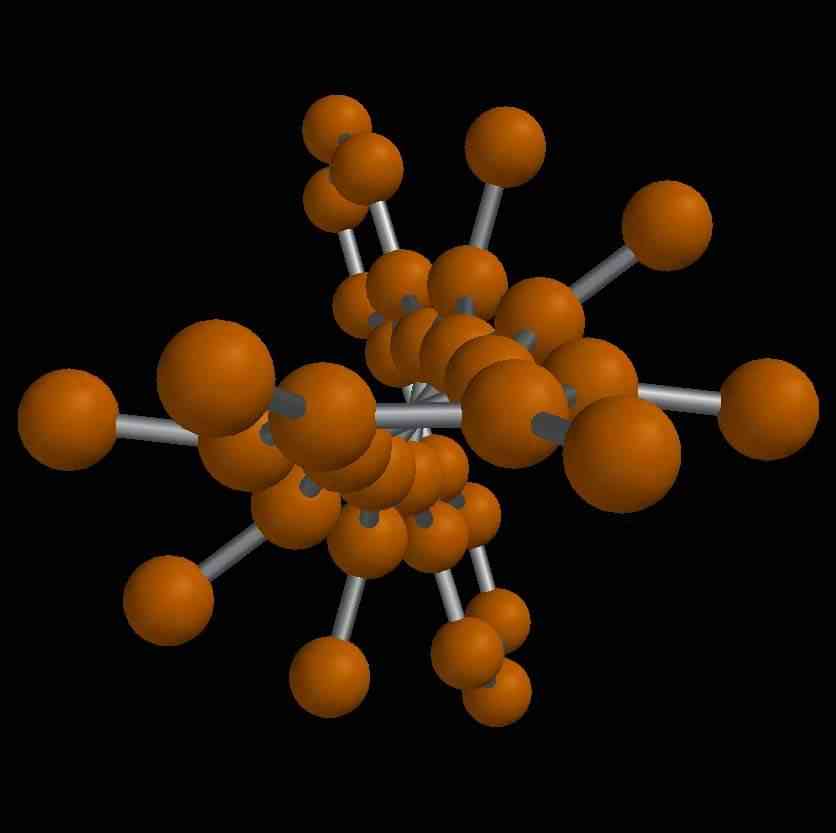
By twisting a PAH he and his team hopes to find out to what extent such molecules may be distorted in this way before they stop behaving chemically and physically as PAHs are expected to behave and start acting like something else. Shape is all-important in most chemical reactions, after all.
Pascal describes twisted polycyclic aromatics as if they are distortions of pieces of a sheet of graphite - a concept that has increased in familiarity with the discovery of the fullerenes.
He and his team managed to twist their otherwise flat molecule by swapping the hydrogen atoms along its outer edges for phenyl groups (C6H5). Phenyl groups are very bulky so if the molecule were flat they would crowd each other out. To avoid this crowding the molecule twists to keep the benzenes as far apart as possible.
The team has calculated that another related compound with extra benzenes fused to the ends will have a 178 degree twist. 'That is our next target,' says Pascal, 'our proposed synthesis is quite short, but depends on a reaction that uses extreme conditions.'
These molecules have two very interesting characteristics: obviously says Pascal they are twisted but secondly the peripheral phenyl groups provide an inert hydrocarbon sheath around a potentially reactive core - like an insulating layer around an electric wire. 'Such a molecule may have superior properties for electrogenerated chemiluminescence, i.e., in an appropriate electrochemical experiment it would glow brightly, and could eventually lead to new light-emitting devices,' says Pascal.
A molecular switch known as cardiac troponin C (cTnC) pulls our heartstrings controlling the beat, but American and Canadian scientists have found it does not in the way they thought. The discovery opens up the possibility of new drugs for heart disease that avoid side effects.
When a nerve impulse reaches the heart it triggers the release of calcium ions. cTnC detects and binds to the ions and in so doing, changes shape pulling the heart muscle into a contraction. This in turn releases the calcium ions from cTnc so it reverts to its original shape and the heart relaxes again ready for the next beat. According to University of Texas biochemist and team member John Putkey, 'Troponin C can be conceptualised as a molecular switch: when calcium is bound, the muscle contracts, when calcium is released, the muscle relaxes.'
cTnC has a similar sequence of amino acids to the related compound in skeletal muscle: sTnC. A University of Alberta team, led by Brian Sykes, has studied the shape of sTnC in the presence and absence of calcium and found that the calcium-bound form of sTnC has an exposed hydrophobic or oily patch on its surface. This site is thought to interact with other regulatory proteins and with any drugs that affect muscle contraction.
It was assumed that the same situation would be present in cTnC. However, Putkey and Sykes used nuclear magnetic resonance spectroscopy (NMR) and recombinant DNA techniques to unfold the three-dimensional structure of cTnC and discovered to their surprise that cTnC does not have such a well-exposed hydrophobic region.
Current drugs assume that cTnC is very similar to sTnC. Putkey and Sykes have shown that this is not true so drug designers should now be able to use this discovery to design more effective drugs that do not interfere with skeletal TnC.