The illuminated late-mediaeval manuscript the Breviarium Mayer van den Bergh was made in Flanders for the Portuguese monarch Manuel I around 1500 and represents an artistic conundrum that Peter Vandenabeele (University of Ghent, Belgium) believes can be solved by analysis. The manuscript has borders filled with flowers and small animals and art historians suspect these were painted by at least three miniaturists. Stylistic comparisons cannot prove it.
Vandenabeele and his colleagues at the Catholic University of Leuven and the Institute for Chemical and Applied Spectroscopy in Dortmund, Germany have combined two analytical techniques, micro-Raman spectroscopy (MRS) and total reflection X-ray fluorescence (TXRF) spectroscopy, to look at the individual pigments in the manuscript. 'Using MRS we can look at the pigment grains separately, while TXRF gives us information about the whole sample,' explains Vandenabeele. First, TXRF determines average elemental composition of tiny pigment grain microsamples. The samples are 100 ng and the technique used leaves no visible trace on the artwork, says Vandenabeele.
Vandenabeele says that the elemental determination provides a fingerprint of the various pigments used. The MRS spectra provide molecular information about the pigments. The researchers know the main 'key-elements' of the pigments, so in many cases the identification of a pigment, can be deduced from the presence of these key-elements identified by TXRF. For example, a lot of mercury in a red sample argues vermilion (HgS) is present. 'As the composition of most inorganic pigments is known, they can often be identified using the key-elements,' explains Vandenabeele. By comparing the MRS spectra with reference spectra the researchers could identify and corroborate the presence of other pigments.
The team discovered several common pigments in the manuscript samples. Azurite, a copper complex, and lapis lazuli (natural ultramarine) were used for blue. Vermilion (mercury sulphide) provided the red, red lead and massicot (two lead oxides) were orange while green was formed from copper sulphates and malachite (copper carbonate-hydroxide). One pink pigment, which lacked inorganic content had an 'organic' Raman spectrum.
Vandenabeele points out that the art historians had clues based on iconography and styling to suggest that artists Simon Bening, Gerard Horenbout and Jan Provoost all worked on the manuscript. By using samples from folios definitely assigned to Provoost, the team compared the pigment mixes used in this with those in the illuminations elsewhere in the manuscript. They found leading clues, such as the very similar elemental percentages in pigment samples from two distinct folios allegedly painted by Provoost. This ties in with the historical evidence, proving the same artist was involved.
Vandenabeele et al., Analyst, 1999, 124, 169
Spiro polymers
The monomeric building blocks in everyday polymers, such as polyethylene, are linked through carbon-carbon bonds, which allows some flexibility of the polymer chain. However, polymer scientists would like to be able to control this flexibility to help them construct rigid nanoscale structures and networks, according to team leader Neil McKeown.
McKeown says that one solution might be to build 'ladder' polymers in which an effectively two-stranded polymer is held together by inflexible bonds between the strands. Such materials are, he concedes, usually difficult to make, limiting their development. However, a sub-class of ladder polymers that uses a spiro linkage might provide the desired rigidity but be more manageable.
Spiro compounds are defined as bicyclic molecules in which the two rings are linked together by a single atom only. The problem with polymerising them is that they produce rigid, crystalline polymers that are insoluble so difficult to process into a useful form. McKeown and colleague Saad Makhseed believe they have overcome this obstacle and have come up with a set of spiro-polyketals that are either 'decorated' with solubilising groups such as large alkyls (C12H25, for instance) or contain irregularities that force the growing polymer chain to kink and reduce unwanted crystallisation.
Their materials are robust and glassy, rather than crystalline, solids stable up to 280 Celsius, which is well above their glass-transition temperature making them suitable for melt processing. McKeown envisages various applications for the new materials as tough thermoplastics and readily cross-linked adhesive materials.
One problem the team is yet to surmount is to make the spiropolymers stable to hydrolysis in solution. 'This could be solved by replacing ketals with thioketals which are much more stable towards hydrolysis,' explains McKeown. Although he adds that limited stability towards hydrolysis could be useful. 'Applications of these materials for drug delivery or as readily degradable polymers could be a possibility,' he says.
Clifford et al., Chemical Communications, 1999, 247.
Supercritical drugs
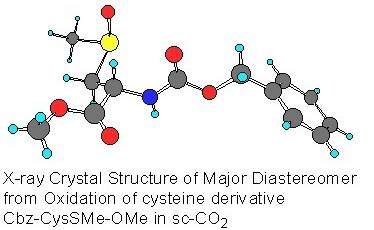
Chris Rayner, and colleagues at Leeds University reported a dramatic pressure dependent enhancement of diastereoselectivity for the sulphoxidation of cysteine and methionine derivatives in Chemical Communications (1999, 247) using supercritical carbon dioxide rather than conventional solvents.
Under high enough temperature and pressure (31 Celsius and 74 atmospheres) carbon dioxide exists as a supercritical fluid - a hybrid half gas half liquid material. Varying the temperature and pressure changes its solvating abilities dramatically. The peculiar properties of SCFs could allow them to replace noxious solvents because they have very low toxicity, are readily available and can be removed from the reaction system easily for recycling.