The alkaline earth metal, beryllium (Be) sits at the top of Group 2 of the chemical elements in the Periodic Table above magnesium (Mg) and alongside Group 1 alkali metal, lithium (Li). It is usually thought of as a divalent metal, bonding to two other atoms and is rarely found in any other form, it is present in the gemstones beryl (aquamarine, emerald) and chrysoberyl. In the free state it is a steel-grey, low-density but strong, but brittle metal. It is acutely toxic, causing chemical pneumonia, and chronic inhalation of dust containing its compounds is an occupational hazard that leads to the lung disease berylliosis.
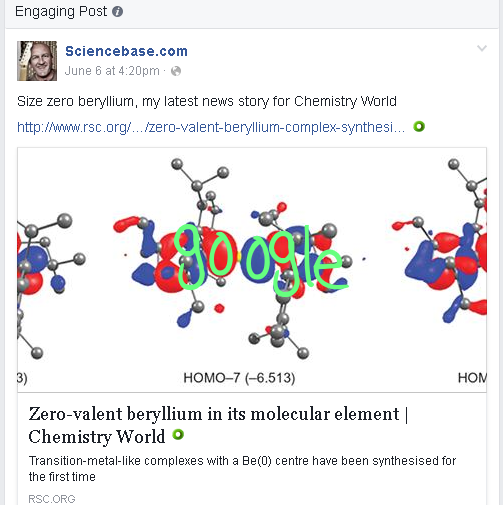
Of course, some chemists love a challenge and recently I wrote about new research into beryllium that has seen the synthesis of a very unusual structure containing the element in a unique position in a compound where it behaves as a molecular element, in the 0 oxidation state, Be(0), rather than the almost ubiquitous divalent Be(II). My news item appeared in the RSC’s Chemistry World magazine and as with many of these structural and bonding odddities attracted the interest of readers far and wide. Last time I checked, it had been shared on social media 1664 times, and its popularity in the Feedly news reader is reported as being four times greater than other updates in the Chemistry World feed.
I shared it to the Sciencebase Facebook page, obviously, and it garnered a lot of interest there too. One reader commented that the structure was reminiscent of a Google Doodle, which I thought was an interesting observation, it’s almost like a molecular Picasso of the search engine’s logo.