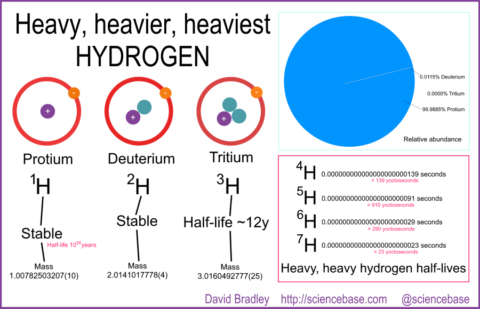
If you learned any chemistry ever, you will most likely know that hydrogen atoms are the simplest atoms – a nucleus comprising a solitary proton with a single electron bound to it. You’ll also presumably know that hydrogen has heavier isotopes, same chemical element, but with at least one neutron to make deuterium, two to make the heavier isotope, tritium. But, Nature Chemistry’s Stuart Cantrill tweeted recently about other isotopes of which there are at least four: hydrogen-4, hydrogen-5, hydrogen-6 and even hydrogen-7. Who knows what the limit might be on heavy, heavy hydrogen, but those later isotopes are very, very unstable.