 This morning, my kids are listening eagerly to the local radio to hear if their school will be closed? Why? Because we’re in England, a few millimetres of snow has fallen, it’s a little chilly, and the nation is in chaos. Airports are shut, driving conditions are almost impossible, and schools are closing across the country. The radio meteorologists are telling us it’s the worst snow “event” since the early 1990s, although it was worse almost exactly twenty years ago as I seem to recall tramping to work in snow when I first moved to Cambridge.
This morning, my kids are listening eagerly to the local radio to hear if their school will be closed? Why? Because we’re in England, a few millimetres of snow has fallen, it’s a little chilly, and the nation is in chaos. Airports are shut, driving conditions are almost impossible, and schools are closing across the country. The radio meteorologists are telling us it’s the worst snow “event” since the early 1990s, although it was worse almost exactly twenty years ago as I seem to recall tramping to work in snow when I first moved to Cambridge.
Anyway, what has all this go to do with salt lowering the freezing point of water? Well, The Highways Agency, which looks after our main roads has had its fleet of gritter lorries spray pink grit across the roads. So, what is it they’re spreading? What is that grit? Well, it’s usually common salt, sodium chloride, but calcium chloride is also used.
Dissolving any compound in another will lower its freezing point slightly. So adding salt to water will lower its freezing point. Scattering salt on the roads therefore allows some salt to dissolve in the surface of snow and ice forming on the roads especially as the early cars put pressure on and briefly melt the very surface of any ice as they roll over it. The salt dissolved means that the temperature has to be that bit lower for water to remain frozen or to freeze further.
The same phenomenon, known to scientists as freezing point depression is the reason why the oceans, in all but the most extreme polar winter conditions, tend not to freeze. The salt content lowers the oceans’ freezing point so that conditions have to be a lot colder to make it freeze.
Freezing point depression
Freezing point depression, like boiling point elevation discussed previously on Sciencebase.com, is a colligative property. This means that the effect depends on the presence of dissolved particles and their number, but not their identity. Anything that dissolves in water will lower its freezing point. It does not have to be salt, indeed it does not have to be ionic like sodium chloride.
It is an effect of the dilution of the solvent in the presence of a solute. It is a phenomenon that happens for all solutes in all solutions, even in ideal solutions, and does not depend on any specific solute-solvent interactions. In other words, the explanation does not lie in some kind of interaction between the solute particles (the dissolved material, sodium chloride, usually used in the salt on roads case) and the solvent (the water ice on the roads) that prevents a solid from forming, it’s an entropy effect:
At the freezing (or melting if you’re heating a solid) point, the solid phase and the liquid phase have the same energy. The chemical potential of each is equivalent. Chemical potential depends on temperature, and at other temperatures the solid or liquid will be favoured over the other phase. At higher temperature, a liquid will exist, at lower a solid.
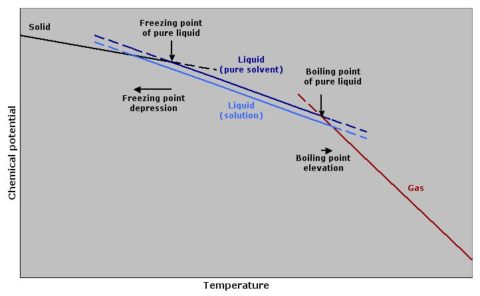
Often, a solute will dissolve only in the liquid and not the solid solvent. If such a solute is added, the liquid is diluted, but its chemical potential does not change. To balance the energy books (you cannot create nor destroy energy), the equilibrium between liquid and solid shifts to a lower temperature, hence freezing point is depression.
Calcium chloride road gritting
So, why choose calcium chloride instead of sodium chloride for gritting roads? Well, both are readily extracted from brine, but CaCl2 has a subtle advantage over NaCl. Not only does calcium chloride shift that energy equilibrium when it dissolves in water so that the freezing point is depressed, it also releases heat when it dissolves in water. This has the distinct advantage of heating the surrounding ice, melting that and allowing more particles of solute to dissolve.
The dissolving process is highly exothermic and in the lab can produce temperatures of around 60 Celsius:
CaCl2 + 2 H2O –> CaCl2·2H2O
Aided by the heat evolved during its dissolution, calcium chloride is also used as an ice-melting compound. Unlike the more-common sodium chloride (rock salt or halite), it is relatively harmless to plants and soil; however, recent observations in Washington state suggest it may be particularly harsh on roadside evergreen trees. It is also more effective at lower temperatures than sodium chloride.
Regardless of the wonders of freezing point depression, at the time of writing, my kids were still huddled around the radio in the desperate hope that they are forced to stay away from school today and build snowmen in the garden and annoy each other with snowballs. And, whether they get to school or not you can rest assured that the “snow event” will continue to plunge Britain into chaos, at least that’s what the BBC will keep telling us.
Wrap up warm, now.