Ship-in-a-bottle catalysts
By: David Bradley
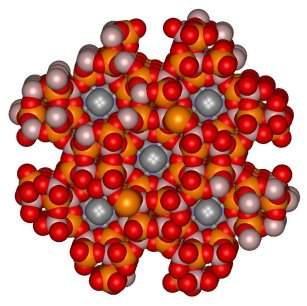 Porous minerals, such as
zeolites, existed in nature for
millions of years before chemists spotted their potential. They have now used
them to sieve out compounds one from another, catalyse reactions, single out
other molecules in sensors and make soap powder work more effectively by mopping
up dissolved metal ions. There are, however, a limited number of these
off-the-shelf minerals in nature so chemists have spent the last few years
looking for ways to tailor their own versions for specific separating, catalytic
and sensors.
Porous minerals, such as
zeolites, existed in nature for
millions of years before chemists spotted their potential. They have now used
them to sieve out compounds one from another, catalyse reactions, single out
other molecules in sensors and make soap powder work more effectively by mopping
up dissolved metal ions. There are, however, a limited number of these
off-the-shelf minerals in nature so chemists have spent the last few years
looking for ways to tailor their own versions for specific separating, catalytic
and sensors.
One approach they have tried is to use small
organic molecules as templates around which a porous mineral structure can be
built. This has proved very successful leading to a whole host of new synthetic
minerals with cryptic names such as the ALPOs, the ZSMs and the UCSBs. Many of
these have useful chemical properties but have not quite achieved the degree of
fine-tuning for particular applications that St Andrews chemist Paul Wright
believes they can. Wright and his team are working on designer
templates which can exert far more control over the ultimate shape and size of
the pores formed in a synthetic zeolite. In 1997, they had found that an easy to
prepare linear diquinuclidinium ion, which is positively charged, flexible and
carries bulky end groups, could be used to template the synthesis of a new
series of porous minerals. They dubbed these new minerals STAs (for St Andrews)
and STA-1 and STA-2 were the first created by heating inorganic aqueous gels
containing the template molecules at 150-200 Celsius. They then took the concept of these tuneable
diquinuclidinium templates to the more shapely triquaternary alkylammonium ions
to create a magnesioaluminophosphate with much larger pores, which they called
STA-5. When the templates are removed these minerals can act as shape selective
catalysts for small molecules. STA-2, for instance, can handle molecules up to 4
� in size, while STA-5 has pores 7� in diameter that give access to much
larger internal cavities.
The critical point, according to Wright, is
that the rational design of the template allowed them to create materials
analogous to the natural zeolites and whose internal pore geometry closely
matches the shape of the included templates. It should therefore be possible to
design structures that are suitable for shape selectivity towards desired
products in chemical reactions such as hydrocarbon conversions and
functionalisation. STA-2, for example, converts methanol with high selectivity
to olefins able to escape from the small pores, which is a potential route to
producing feedstock ethylene and propylene.
Spurred on by their successes, Wright's team
set about designing other templates to make minerals with different pore shapes
and sizes that might have other novel catalytic and sieving properties.
Wright points out that until now the shape and
size of the pores is often dominated by the organic template and this is
certainly the case with the latest template to be exploited by his team. The
researchers have used a macrocyclic molecule,
1,4,8,11-tetramethyl-1,4,8,11-tetraazatetradecane, to create a synthetic
magnesioaluminophosphate mineral STA-6. The pores, explains Wright, have
'remarkably high symmetry and form a very close fit around the azamacrocycle.'
The template molecule itself also possesses functionality - inorganic chemists
have long studied their properties in cation complexation and catalysis.
The crystals of STA-6 formed during synthesis
are microscopic and require a scanning electron microscope to see their
tetragonal prismatic shape. However, crystal size does not matter in this
instance as the structure of STA-6 was readily determined by synchrotron X-ray
diffraction.
When the researchers blasted the mineral at 550
Celsius in a stream of oxygen they could remove all traces of the organic
template. The hollowed out mineral is all that is left behind with its
tetrahedrally connected framework and its large symmetrical pores intact The
result is a catalytic solid, but points out Wright, it might be better to leave
it in and use its functionality for cation exchange and catalysis.
While many synthetic minerals have their own
catalytic abilities, Wright's team is also collaborating with St Andrews' David
Cole-Hamilton to incorporate or trap transition metal complexes within the pores
of their minerals. This means that catalysts that normally have to be dissolved
with the starting materials to produce a homogeneous system can be kept in a
separate phase but remain just as active as the reagents react within the pores
of the mineral. This is known as the 'ship-in-a-bottle' method and was
originally developed by N Herron of Dupont (Wilmington, DE) and later by Thomas
Bein at Purdue University in West Lafayette, Indiana. The catalyst is made
within the pores of the mineral and cannot escape during use because it is too
large or the wrong shape. The mineral with its catalyst payload can simply be
filtered off from the product once the reaction is complete, for re-use.
Wright's macrocycle template, he says might be the ship itself in the catalytic
bottle.
P.A. Wright et al., Angew. Chem. Int. Ed. Engl., 1997, 36, 81.
V. Patinec, P. A. Wright, P. Lightfoot, R. A. Aitken and P. A. Cox, Dalton
Trans., 1999, 3909.
R. E. Morris and P. A. Wright, Chem Ind, 1998, 256.
P.A.Wright, Chem. Mater., 1999, 11, 2456.
Back to the index
Nothing lasts forever
If left at room temperature dehydrated carbonic acid (the
stuff that makes rain and blood acidic) will last for 0.18 million years,
according to Austrian and British theoretical chemists, which is quite a long
time for a supposedly reactive and unstable chemical.
Klaus Liedl and his colleagues at Innsbruck
University working with a team at Queen Mary and Westfield College London
believe the secret lies in the low rate at which the molecule of carbonic acid
in a sample decays to release water and carbon dioxide. But, once the first
drops of water are formed and bubbles of CO2 released the whole process quickly
accelerates leading to the breakdown of the whole sample.
The hydration of carbon dioxide is a
fundamental biochemical reaction - being at the heart of the outgoing stage of
respiration and other key metabolic processes. Carbonic acid the partner of CO2
in these processes also finds itself in a critical position in inorganic
reaction schemes in nature and the laboratory. Despite its prominence, however,
synthesising the compound has been difficult and even spotting the raw H2CO3 as
a reaction intermediate has been hampered by its rapid decay. There was even the
notion that the pure compound didn't exist.
The team applied various theoretical modelling
techniques to carbonic acid's decomposition kinetics to try and discover why it
had remained such an elusive character. Their startling results demonstrate that
pure carbonic acid in the gas phase is in fact a very stable compound with a
half-life of 180,000 years! Their calculations however show that should
just a single molecule of water become embroiled with H2CO3, for instance, if
one molecule of acid breaks down in a sample, then a self-catalysing process
takes over the leads to the ultimate demise of the sample. One potential application of the research is in
the study of interstellar chemistry. If, as turns out to be the case, pure
carbonic acid is quite stable, then it may be involved in all kinds of cosmic
chemistry perhaps even in the formation of fullerenes and their ilk in the
interstellar dust.
Angew. Chem. (Engl. Edn.), 2000, 39, 891
Now you see it..
Back to
the index
Acetylene direct
A room temperature method for converting natural gas into the
more useful organic acetylene has been developed by Japanese chemists. The
technique uses an electric discharge rather than the 1273K pyrolysis
temperatures normally required for such a direct conversion making it a simpler
and cleaner process for utilising non-oil-derived chemical feedstocks.
Shigeru Kado and his colleagues the University
of Tokyo point out that methane is usually so stable that the only way to
directly convert it into higher hydrocarbons is to use a combination of high
pressure, high temperature or a suitable catalyst in a plasma discharge. With an
NaY zeolite catalyst high yields of C2 hydrocarbon can be produced but while
simply heating to the pyrolysis temperature is effective at conversion the blast
usually decomposes much of the product down to carbon.
The Japanese team wanted to avoid the use of
catalysts and high temperatures. ' We are using a pulse discharge lasting less
than 1 microsecond which means only electrons are accelerated and other species
remain in a non-equilibrium state, keeping the reaction at room temperature in
the gas phase, and the products are not decomposed,' explains Kado.
They built a flow reactor into which methane
could be pumped at atmospheric or higher pressure for greater product throughput
and ambient temperature. At each end of the flow chamber they placed a steel
electrode. With pure methane being fed in they pulsed the gas at up to 60 Watts
and obtained greater than 90% conversion to acetylene in the absence of
catalyst. There was little ethylene or ethane by-product. While conversion to
yet higher hydrocarbons such as prop-1-yne and buta-1,3-diene was less than 1%.
However, there was some soot deposition on the
electrodes and the inside of the reaction chamber, which Kado says ultimately
blocks the reaction. A 5:1:4 mix of methane:oxygen:argon helped reduce soot
formation.
They found that with this gas feed there was
not much change to the overall reaction products with the proportions of
side-products remaining fairly constant although there was of course, the
additional side products of carbon monoxide and dioxide. But, the reaction of
the soot with the oxygen under discharge from which these side-products
originate actually stabilises the discharge itself so boding well for a longer
running reaction.
The team points out that they can avoid carbon
monoxide as a by-product by using methane mixed with hydrogen gas at 1:4. This
also helps stabilise the discharge and up to a limit achieves similar conversion
selectivity.
Kado says the team hopes to combine the
discharge approach with catalysis to develop the method for activation of
methane to methanol, for instance.
Chemical Communications, (1999, 2485).
 Porous minerals, such as
zeolites, existed in nature for
millions of years before chemists spotted their potential. They have now used
them to sieve out compounds one from another, catalyse reactions, single out
other molecules in sensors and make soap powder work more effectively by mopping
up dissolved metal ions. There are, however, a limited number of these
off-the-shelf minerals in nature so chemists have spent the last few years
looking for ways to tailor their own versions for specific separating, catalytic
and sensors.
Porous minerals, such as
zeolites, existed in nature for
millions of years before chemists spotted their potential. They have now used
them to sieve out compounds one from another, catalyse reactions, single out
other molecules in sensors and make soap powder work more effectively by mopping
up dissolved metal ions. There are, however, a limited number of these
off-the-shelf minerals in nature so chemists have spent the last few years
looking for ways to tailor their own versions for specific separating, catalytic
and sensors.