Microwaved Solid Acids
A new green route to pyrrole and thiophene derivatives has been developed
by Spanish researchers. These compounds are extremely useful in making
bioactive materials, organic semiconductors and addressable gene chips but
converting the basic skeleton heteroaromatic structures into more useful
compounds tends to be a hazardous and wasteful procedure. Chemists
 have
been looking for a safer, more efficient approach for years.
have
been looking for a safer, more efficient approach for years.
Juan Palacios and his colleagues at the Universidad de
Extremadura in Badajoz, Spain have found that they can carry
out homo-Diels-Alder reactions under solvent-free conditions using
inexpensive and innocuous mineral solids as catalysts. They previously
devised an approach to competitive cycloaddition and Michael-type
reactions on furans and with those results in hand, they then turned to
pyrroles and thiophenes. They now believe they have found an effective
method of producing functional pyrroles and thiophenes that avoids solvent
and is speeded up using microwaves. The process exploits safe and reusable
solid acids and can be modified to allow them to accommodate one or two
alkyl groups on the starting materials.
By using a solid acid, such as the clay montmorillonite K10
Palacios and his team can side step the problems of handling acid catalyst
solutions, which are normally required for condensation reactions of
pyrroles and thiophenes. They have now carried out fast and efficient
condensation reactions with enones, such as N-phenylmaleimide, and
alkynes to make mono- and bis-alkylated products.
Palacios and his colleagues have found that they can react both
pyrrole and thiophene with the likes of maleic anhydride, methyl vinyl
ketone, N-phenylmaleimide and dimethyl acetylenedicarboxylate under
such reaction conditions using a laboratory microwave reactor. They point
out that a domestic oven might alternatively be used with equally good
results.
Palacios points out that with only a few exceptions, most of their
products were obtained as homogeneous oils rather than crystalline solids
but gave satisfactory combustion analyses. The team is currently
investigating the reaction to determine whether it follows a concerted or
stepwise pathway, a better understanding of this will allow them to fine
tune the reaction more effectively.
The team reports its results in more detail in Green Chemistry (DOI: 10.1039/b008795p)
Elemental Discoveries
March 2001, Issue 39
Combined acceleration
British chemists are using NMR spectroscopy to sort the wheat from the
chaff in their combinatorial libraries. The approach could allow them to
more quickly home in on effective host molecules for novel sensors.
The great bugbear of combinatorial chemistry, whether it is used to
generate libraries of putative drugs, catalysts or other useful compounds,
is picking out the potentially useful members from the sometimes vast
compound libraries.
Nuclear magnetic resonance spectroscopy can come to the rescue,
according to Philip Hodge and Gareth Morris and their colleagues at the
University of Manchester. They are using NMR to help them pick out the
best host molecules from an array of hopefuls for use in analytical sensor
applications.
The team uses high-resolution diffusion-ordered spectroscopy (HR-DOSY), which is a multi-dimensional version of NMR, which disperses signals for the members of the library according to an additional criterion: their diffusion coefficients. Normally all the members will diffuse relatively rapidly. However, when a soluble polymer, with an added side-group of interest, is added to the combinatorial array, each member of the array that binds to the functionality on the polymer diffuses more slowly than before. The magnitude of the shift indicates the strength of the interaction. The spectrum of the most tightly bound molecule can then be plotted.
The team is now extending the approach to finding recognition hosts for amines, amides and peptides using libraries of macrocycles.
Find out more in Chem Commun (DOI: 10.1039/b008412n)
Elemental Discoveries
March 2001, Issue 39
Chemical triangulation
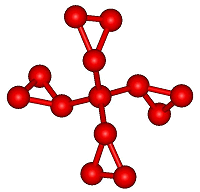 A beautiful and unique molecule in which four cyclopropyl rings are
attached to a single central carbon atom has been synthesised by Armin de
Meijere of the Georg August University, Göttingen, Germany and his
colleagues.
A beautiful and unique molecule in which four cyclopropyl rings are
attached to a single central carbon atom has been synthesised by Armin de
Meijere of the Georg August University, Göttingen, Germany and his
colleagues.
Neither of the two commonly used approaches to percyclopropylated
compounds worked so his team opted for a twofold cyclopropanation of
dicyclopropyldiethenylmethane obtained from ethyl
3,3-dicyclopropylacrylate as the starting material to make
tetracyclopropylmethane.
Its X-ray analysis showed this peculiar hydrocarbon to have a
propeller shape with S4 symmetry. The team is now in pursuit of the even
more congested tetra-tert-butylmethane.
More details can be found in Angew. Chem. 2001, 40, 180.