Wind turbines, photovoltaic power cells, wave energy, porous hydrogen storage composites for fuel cells, carbon sequestration, nuclear, even the idea of damming the Red Sea for a massive hydroelectric power plant are among the high-tech approaches being developed in the battle to reduce our collective size 9 carbon footprints to mere tiptoes.
Saving energy and reducing emissions does not have to be about high tech and macro engineering. The developed world is unlikely ever to give up its dependence on personal motor transport, frequent and pointless air travel, patio heaters, high-definition DVDs, hot and cold running water, and countless other energy-intensive luxuries unless someone actually physical pulls the power plug.
However, for millions of people in the developing world, who may not even be aware of the problems we face with iPhone tariffs, double booked business flights, and lost Facebook friends, life is hard at a much more fundamental level. Aside from poverty, unreliable water supply, malnutrition and disease, even very basic needs are not met, such as keeping warm and dry in freezing mountain villages with no access to heated spa pools and acrylic nail extensions, and wireless internet access to refresh the contents of your Kindle.
D. Buddhi of the Thermal Energy Storage Laboratory at Devi Ahilya University in Indore, India, Atul Sharma of the Department of Mechanical Engineering at Kun Shan University, Tainan, Taiwan, ROC, and S.D. Sharma of UAE Innovations Center in Ras Al Khaimah, UAE have turned to a perennial problem of those without the comforts of central heating and doubly glazed windows – keeping one’s feet warm.
The researchers have designed and tested three foot warmers that can store up solar energy during sunshine hours and then be used during cold evenings as a sustainable alternative to electric heaters. The optimum temperature, the team found in their trials, was just above body temperature. With this in mind, commercial grade lauric acid (which has a melting point 42.2 Celsius and a latent heat of fusion of 181 kilojoules per kilogram was used as the latent heat storage material in their PCM (phase change material) designs. Basically, the material inside melts in the sun and then once the sun goes down the material begins to solidify giving off enough latent heat as it does so to raise the temperature of the container to about 40 Celsius.
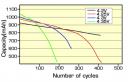
They carried out experiments (details are reported in the International Journal of Global Energy Issues) during a winter season to study how well the PCM units performed in the sunny but cold winter climate of northern India. The devices could easily reduce reliance on costly 1 kilowatt electric heaters for keeping the legs and feet warm; and although PCM units do not have the benefit of creating circulating warm air in a room, much of that energy is wasted anyway.
So, could a solar-powered foot warmer save the planet? Perhaps not, at least not until those in the developed world abandon their predilection for vehicle climate control and optical mice. However, alongside other simplified technologies such as solar cookers, arsenic-removing water filters, and clockwork radios, they could at least make a significant difference to the cost of living of people in the poorer parts of the world without compromising their quality of life. Moreover, those in frozen climes will at least be able to keep their feet warm while pondering shag pile carpets and remote control storm shutters.