Benzimidazole opioids, also commonly known as nitazenes, were first synthesised by CIBA Pharmaceuticals in the 1950s as putative alternatives to morphine and heroin for use as strong painkillers. They have never made it into use in clinical medicine because the risk of addiction, respiratory depression, and death in use is too high.
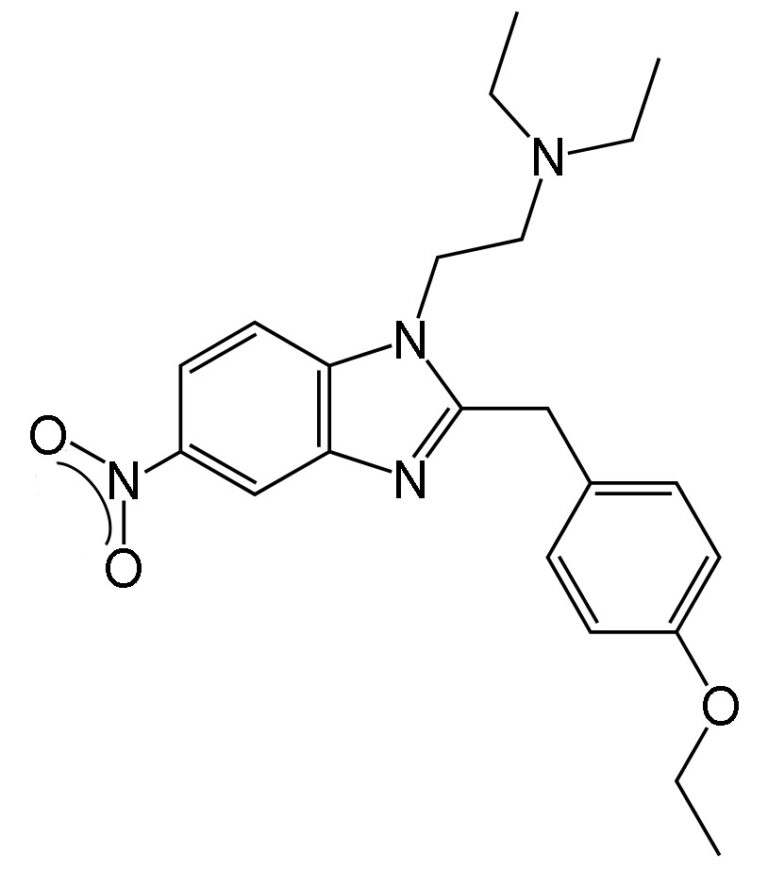
The compounds are classified as opioid New Psychoactive Substances (opioid NPS). Their mode of action is to bind to the brain’s mu-opioid receptors, but their unique structure means that some examples are several hundred times more potent than morphine and stronger even than fentanyl.
The changing heroin market led to the emergence of nitazenes as drugs of abuse in the early 2020s if not earlier. They pose a major new problem for public health and law enforcement.
Nitazenes were first in the UK news in 2021, when an 18-year-old patient was treated for overdose. Since then, there has been a rise in nitazene abuse with more than fifty overdose deaths and perhaps many more that remain inconclusive. Several nitazenes are, as of this week, defined as Class A drugs in the UK.
On a point of chemical semantics, opiates are chemicals derived from opium or poppy straw. They are alkaloid compounds naturally found in the opium poppy plant, Papaver somniferum. The archetypal opiate is the compound morphine. The analgesic pro-drug codeine is converted in the body to morphine. Heroin (diamorphine) is extracted from the dried latex of the poppy and has a very similar chemical structure to morphine.
The term opioid is used to designate any substance, natural or synthetic, that binds to the opioid receptors in the brain. So, this includes morphine and heroin, but also compounds that are not chemically related to the opiates such as fentanyl, which is a piperidine, and the nitazenes, which are benzimidazoles. There are many different benzimidazoles used safely in pharmaceuticals for treating high blood pressure, fungal infections, parasitic worms etc.