 The Periodic Table of the elements is a fascinating icon of science. It is incredibly useful and has been exploited and sexploited too in the form of a periodic table of yoga and a sexy PT. It has also been hacked apart, cut and paste into different formats, created as illuminated wall cases, woodworked into furniture, spiralled, spherized, and generally rebuilt in almost every imaginable way ever since Mendeleev first dreamed of laying out his elemental cards according to the periodicity of elemental properties.
The Periodic Table of the elements is a fascinating icon of science. It is incredibly useful and has been exploited and sexploited too in the form of a periodic table of yoga and a sexy PT. It has also been hacked apart, cut and paste into different formats, created as illuminated wall cases, woodworked into furniture, spiralled, spherized, and generally rebuilt in almost every imaginable way ever since Mendeleev first dreamed of laying out his elemental cards according to the periodicity of elemental properties.
Now, in an effort to inspire chemists to reconsider the foundations of the periodic table, chemical philosopher Eric Scerri of the University of California, Los Angeles, is building a new way to classify the chemical elements one step at a time.
Writing in the latest issue of the Journal of Chemical Education (PDF 2008, 85, 585-589), Scerri explains how the periodic table initially arose from the discovery of atomic weight triads but he now suggests that chemists should recognize the fundamental importance of atomic number triads.
This sea change in elemental attitude might enhance the periodic table by classifying the elements at a fundamental level as basic substances. As such, he and his colleagues have developed a new version of the “left-step” periodic table, which looks very different from the conventional PT. In the new layout, with its step-like pattern actinides and lanthanides are no longer relegated to a standalone box, but form the first step of the PT.
Climbing right to the transition metals (Fe, Mn, Ir, Sg et al) on the next step and then to the non- and semi-metals, such as boron carbon, oxygen, silicon etc and finally a step in which the halogens (fluorine, chlorine…), noble gases (neon, xenon…), alkali metals (potassium, sodium…) and alkaline earth metals (beryllium, calcium…) form the final highest step on the right. Hydrogen tops the halogen column and helium crowns the noble gases rather than acting as the outer beacons as with the conventional layout. (Click the graphic for a clearer, full-size view).
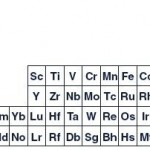
“The left step table has been around for some time,” Scerri told me, “but I am modifying it to accommodate two atomic number triads which would otherwise be absent. They are He, Ne, Ar which ceases to exist as a triad in the usually encountered left-step table and H, F, Cl which does not exist either in the conventional medium-long form table or the usually encountered left-step table.”
In the grander scheme of things, whatever form the Periodic Table takes in the future matters not to those of us who sing, so we end with a song, the periodic table song from Tom Lehrer (who was 80 on April 9, 2008 and gets a mention in the Official Google Blog this week), known simply as The Elements.