 As it is a holiday across the UK today, there is probably little need to warn Brits of the dangers of the sun’s rays, it’s usually so cold and wet, that the chances of frostbite and rust are much higher than sunburn. That said, summer is on its way and a paper in the latest edition of the Lancet medical journal warns that sunscreen and light cotton clothing are simply not enough to protect you from skin cancer caused by exposure to ultraviolet light from the sun.
As it is a holiday across the UK today, there is probably little need to warn Brits of the dangers of the sun’s rays, it’s usually so cold and wet, that the chances of frostbite and rust are much higher than sunburn. That said, summer is on its way and a paper in the latest edition of the Lancet medical journal warns that sunscreen and light cotton clothing are simply not enough to protect you from skin cancer caused by exposure to ultraviolet light from the sun.
Instead, the paper’s author suggest that anyone who dares to partake of the great outdoors should wear heavy cotton clothing such as denim, wool, or polyester, to block out those damaging rays. But, should this advice be well taken? Is the sun really to blame for the apparent increased incidence of skin cancer we hear reported or could it be that our car and desk bound sedentary lifestyles in which most people barely see the sun except on their foreign holidays are more to blame for compromising our immune systems and making us more susceptible to skin and various other forms of cancer.
We’ve covered this issue previously in Sciencebase, recent evidence points to a lack of vitamin D (manufactured in the skin during sunlight exposure) as being a much higher risk factor for various cancers than sun exposure itself.
The Lancet Review authored by dermatologist Stephan Lautenschlager of Triemli Hospital, in Zurich, Switzerland, analysed the various sun protection strategies used around the globe. “Wearing sun protective clothes and a hat and reducing sun exposure to a minimum should be preferred to sunscreens,” the team writes, “Often this solution is deemed to be unacceptable in our global, outdoor society, and sunscreens could become the predominant mode of sun protection for various societal reasons, for example healthiness of a tan, relaxation in the sun.”
The Review says that linen and loosely woven cotton represent less effective sun protection and that tightly woven, thick garments made of denim, wool or polyester offer the best protection; not exactly the kind of clothing anyone but heavy metal fans would want to wear on a scorching hot sunny day.
The paper points out that several studies have shown that sunscreen protects against acute UV skin damage and non-melanoma skin cancer, it is not known whether sunscreen stops melanoma developing. And, perhaps therein lies the rub, the connection between sunlight exposure and skin cancer itself is not as cut and dried as some commentators suggest.
“Suggesting wearing denim in hot weather is I think so stupid – it is very uncomfortable. Special clothing is not needed in the UK – on rare very hot days it is better simply to seek the shade – after some exposure to get your dose of D without burning, says Oliver Gillie of London-based lobby group Health Research Forum. He points out once more, that insufficient exposure to sunlight could be doing us more harm than good in terms of increasing cancer risk because of a lack of vitamin D.
“A link between heart disease and insufficient vitamin D is emerging,” he told Sciencebase, “and the National Heart Forum is interested in this aspect of the debate.” Given that until now the sunlight and skin cancer debate has essentially been a Cancer Research UK monopoly, it will be interesting to see how the heart charity approaches the issues.
There have been numerous other research developments that have not seen the media light of day. “There are links with infectious disease,” adds Gillie, “Vitamin D is important for maintaining immune system resistance a fact well-known to those treating tuberculosis a century ago and well before the advent of antibiotics.”
Cancer Research UK says that 90% of melanoma is caused by sun exposure. “This is a very contentious figure,” Gillie points out, “and is disputed.” He adds that it could be that as few as one in ten melanoma cases are caused by sun exposure. “Poor immunity is a big factor in melanoma and people who are on immune suppressing drugs e.g. transplant patients are at high risk,” he says.
Such observations certainly cloud the picture of sun exposure as the big skin cancer killer. He also points out that purported mechanisms for DNA damage and thence skin cancer formation based on the photochemistry of DNA itself no longer stack up because it is now known that melanin, the pigment that gives rise to a tan is a protective agent against the very mutagenic molecules thought to form on sun exposure. Indeed, Raymond Barnhill and colleagues writing in the Journal of the National Cancer Institute (2005, 97, 195-199) found that people with melanoma survive longer if they have more sun exposure. This is doubly ironic in that post-treatment melanoma patients are usually advised to stay out of the sun.
If the British weather is kind for once this Bank Holiday Monday, it will pour sunshine down on all of us. So, leave the denim at home, unless you are a heavy metal fan, and make sure your icecream doesn’t melt while you are sunning yourself.
 The Wolfram Demonstrations Project launched this week and represents what the developers describe as “a major new resource for research and education”. Well, that’s as maybe, but what is it? The project was first conceived by Stephen Wolfram creator of the Mathematica software that as its name suggests allows computers to produce visualisations of mathematical concepts.
The Wolfram Demonstrations Project launched this week and represents what the developers describe as “a major new resource for research and education”. Well, that’s as maybe, but what is it? The project was first conceived by Stephen Wolfram creator of the Mathematica software that as its name suggests allows computers to produce visualisations of mathematical concepts.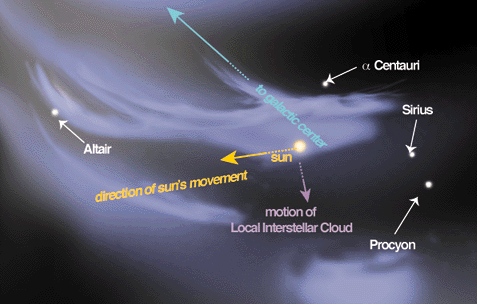 Sending astronauts up to our nearest star to reignite the Sun, the premise of sci-fi movie Sunshine, is truly the least of our problems when we are currently faced with global climate change, global terrorism, and global economic collapse. Nevertheless, astronomers are concerned about recent findings regarding the hot gas surrounding our star and its stellar neighbours. Put simply they cannot find them.
Sending astronauts up to our nearest star to reignite the Sun, the premise of sci-fi movie Sunshine, is truly the least of our problems when we are currently faced with global climate change, global terrorism, and global economic collapse. Nevertheless, astronomers are concerned about recent findings regarding the hot gas surrounding our star and its stellar neighbours. Put simply they cannot find them.