Joana Mendonça and Rui Baptista of the Centre for Innovation, Technology and Policy Research at the Technical University of Lisbon, working with Paulo Conde of Solvay in Brussells, Belgium, have examined how innovation occurs within the chemical industry, by examining the processes and activities undertaken by the Portuguese branch of a multinational chemical company. They have looked at the company’s formal innovation process and from that they have gleaned a map of the knowledge bases used in the search for innovative, new products.
Europe is a major player in the global chemical industry, but recently supply has begun to shift towards the Asian and Middle East markets. Demand from these regions is increasingly rapidly but their own fast-developing industries may not face the same high production costs and strict environmental regulations that increasingly make Europe a less attractive investment.
Couple this socioeconomic and geographical shift in production with a fall off in R&D spending in Europe and the exodus of skilled labour and on the surface it appears that chemical industry innovation within Europe is on the wane. Faced with this prospect, Mendonça and colleagues suggest that it, “is of crucial importance to analyse its processes within the chemical industry.”
Their analysis of the chemical industry has allowed them to produce a map showing the spread of the industry’s widely distributed knowledge bases and to demonstrate how knowledge flows between them and how it is used. They have found that the ability to generate value-creating knowledge is concentrated in the early stages of the industry’s lifecycle regardless of region. In contrast, the Portuguese industry is mostly concentrated on activities that have already reached maturity and, in some cases, are in decline rather than looking to innovation. “Owing to this asymmetry, disembodied knowledge flows are difficult to create, and other types of relationship should be pursued,” they suggest.
They also point out that multinational companies tend to rely strongly on internal improvements and do not seek new knowledge from outside sources that might lead to profitable innovation or improvements in efficiency. Indeed, any innovative activities that take place are actually focused on preventing “unwelcome surprises and to minimise risk” as opposed to facilitating the kin of “freewheeling, imaginative, and risk-taking approach that characterises entrepreneurship”.
All is not lost, add the researchers, “large multinational companies can have a decisive role in the innovation process by providing their market expertise to entrepreneurs and the case study presented shows a path other companies may follow.”
The original research paper, “A map of the knowledge bases for the chemical industry” can be found in the current issue of the International Journal of Technology, Policy and Management (2007, 7, 245-262)
 Does eating chocolate give you a headache? What about red wine? Cheese, perhaps? Yes, well read on to find out how a space-age detector developed to look for signs of life on Mars could soon become the kitchen gadget of choice for anyone who suffers a painful reaction to their food.
Does eating chocolate give you a headache? What about red wine? Cheese, perhaps? Yes, well read on to find out how a space-age detector developed to look for signs of life on Mars could soon become the kitchen gadget of choice for anyone who suffers a painful reaction to their food.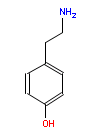 “Some foods have more biogenic amines than others,” explains Mathies, “but you cannot tell in advance because they aren’t listed on the food labels.” Even a single glass of wine has been known to trigger elevated blood pressure, heart rate and headaches in some people, he adds. He suggests that food manufactures and wine producers should be obliged to list biogenic amine content in their products by law. Although if they did, then this would preclude the need for the test kit, I assume, so the research team could concentrate on sending it to Mars instead.
“Some foods have more biogenic amines than others,” explains Mathies, “but you cannot tell in advance because they aren’t listed on the food labels.” Even a single glass of wine has been known to trigger elevated blood pressure, heart rate and headaches in some people, he adds. He suggests that food manufactures and wine producers should be obliged to list biogenic amine content in their products by law. Although if they did, then this would preclude the need for the test kit, I assume, so the research team could concentrate on sending it to Mars instead.
 This issue, I also report on how detecting the differences between young and old hair using Raman spectroscopy is now possible, even if the hair is highly pigmented. You can find out more about that here.
This issue, I also report on how detecting the differences between young and old hair using Raman spectroscopy is now possible, even if the hair is highly pigmented. You can find out more about that here. Plain or Vanilla
Plain or Vanilla


