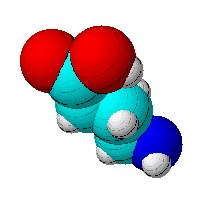
In chemistry, hydrophobicity (from the combining form of water in Greek hydros and for fear phobos) is the physical property of a molecule that is repelled from water. Hydrophobic molecules tend to be non-polar and thus have a greater affinity for other neutral molecules and non-polar solvents. Hydrophobic molecules in water often cluster together forming tiny bubble-like structures known as micelles.
The opposite of hydrophobic is hydrophilic. A hydrophilic substance, from the Greek hydros for water and philia love, is a molecule or other molecular entity that is attracted to, and tends to be dissolved by, water. A hydrophilic molecule is one that has a tendency to interact with or be dissolved by water and other polar substances including solvents; the interactions are more thermodynamically favourable.
Water always was a slippery character. Now, scientists at Pacific Northwest National Laboratory have made an incredible thin layer of water, just a single molecule thick, that sits on a slab of platinum metal and refuses to freeze. Strictly speaking, the monolayer held at just 60 Kelvin becomes hydrophobic and will not allow ice crystallites to form on its surface.
What’s that you say, “hydrophobic water”?
Denial doesn’t get any more profound than that, even if we are talking about molecules.
According to Physics News Update: “Weaker bonding results in a “classic” hydrophobic state, in which the water merely balls up immediately.” So, it’s a balls up, is it? That explains a lot.
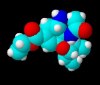 Roche is allegedly struggling to keep up with unprecedented demand for its antiviral Tamiflu in light of the massive media scaremongering that is going on globally thanks to the emergence of the H5N1 strain of bird flu. Taiwan already intends to stockpile a generic version of the drug oseltamivir with or without Roche’s permission. Currently, oseltamivir is synthesised from shikimic acid, which is obtained from the star anise fruit. The total synthesis takes at least ten steps, but chemists are working on simpler approaches.
Roche is allegedly struggling to keep up with unprecedented demand for its antiviral Tamiflu in light of the massive media scaremongering that is going on globally thanks to the emergence of the H5N1 strain of bird flu. Taiwan already intends to stockpile a generic version of the drug oseltamivir with or without Roche’s permission. Currently, oseltamivir is synthesised from shikimic acid, which is obtained from the star anise fruit. The total synthesis takes at least ten steps, but chemists are working on simpler approaches.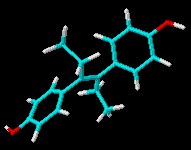 Dan Lednicer offered us a guest editorial some time ago that mentioned the ill-fated drug diethyl stilbestrol (DES) and the toxicity of this and other compounds. Here’s the 3D chemical structure of DES for recent visitors to Sciencebase who were looking for it!
Dan Lednicer offered us a guest editorial some time ago that mentioned the ill-fated drug diethyl stilbestrol (DES) and the toxicity of this and other compounds. Here’s the 3D chemical structure of DES for recent visitors to Sciencebase who were looking for it!