 Does nanotechnology pose a threat to humanity on a par with the threat we face from genetically modified (GM) foods? That is the question asked by various lobbying groups from the verdant greens to the confused consumers. But, governments, academics and commercial bodies see the issue from an entirely different perspective and are asking, will the nanotechnology industry face the same outpouring of hostility as that caused by genetically modified (GM) foods? The kind of outpouring that stymies and often destroys research that could benefit us all.
Does nanotechnology pose a threat to humanity on a par with the threat we face from genetically modified (GM) foods? That is the question asked by various lobbying groups from the verdant greens to the confused consumers. But, governments, academics and commercial bodies see the issue from an entirely different perspective and are asking, will the nanotechnology industry face the same outpouring of hostility as that caused by genetically modified (GM) foods? The kind of outpouring that stymies and often destroys research that could benefit us all.
Nanotechnology, the application of objects and structures that are very small, usually less than 100 nm in diameter is growing rapidly. But aside from the bizarre imaginings of those who originally pioneered the term, this new science is really not much different from what chemists, materials, scientists, and physicists have always done and that is to work with particles, molecules, and other species that just happen to be individually very small. The advent of scanning microscopy techniques has given us a closer look at the structure of atomic clusters, supermolecules and nanomaterials, and new techniques for handling materials at the very small scale or creating species that have novel functionality at this level are emerging all the time. However, the nano, meaning a billionth (in this case a billionth of a metre) is really nothing new, it simply defines the size limits.
Much of the nano that hits the press is nothing of the sort, remember those little cogs and pistons etched in silicon? And, what about the tiny bots that would defur arteries? They are a thousand times bigger than truly nanoscopic objects and the likes of liposomes, which the cosmetic industry briefly marketed as nanotechnology are really nothing of the sort, they are simply supramolecular chemistry given a trendy name.
According to a Leicester University press release this week touting a talk to be
given in May by new media expert Rachel Gibson is studying the nature of the growing online nanotech debate and will present her latest findings to the annual meeting of the International Communication Association in May 2007. The release says that, “at such scales, the ordinary rules of physics and chemistry no longer apply.” This is baloney! The normal rules of physics and chemistry very much apply, at almost every scale. In fact it is at the nanoscale that we can see the beauty of the normal rules of physics and chemistry in action – the quantum effects, such as tunnelling, the formation of electrostatic, non-covalent bonds, the non-linear behaviour that continually astounds but never breaks those laws. These are the normal rules of the physical sciences. There is no mystery about nanotechnology.
That said, the promise of nanotechnology is as big as the grants researchers are receiving should they happen to splash a few “nanos” into the application forms. Novel materials and composites indeed do have huge potential medicine, engineering, communications, transport, even the home.
But, the inevitable questions from environmental groups and ethicists are repeatedly raised. The outpourings of certain British Royals concerning “grey goo” and other garbage do not lend themselves to a sensible debate either. And, yes, there should be a debate if we are finding such unique physical behaviour on this scale that it represents some kind of threat. But, the idea of self-replicating nanobots that chomp their way across the planet, is quite literally, a Michael Crichton plot, I believe, rather than a serious prediction about where nano is heading.
Gibson, however, hopes to examine the efforts of the scientific community to engage with the various social concerns and to assess the extent to which the GM lessons are being learned as nanotechnology (more realistically at the moment mere nanoscience) diffuses into the public consciousness.
She is working with colleagues from the Australian National University, to develop software to study the structure, evolution and implications of the online hyperlinking activities of political and social organisations. The Virtual Observatory for the Study of Online Networks (VOSON) (http://voson.anu.edu.au) thus provides social scientists with the means to study the success of opposition of groups. Watching the evolution of the debate online offers a new way of studying this question, she says, particularly as many of the groups active on the issue are enthusiastic users of the Web.
“This project demonstrates the growing interest among social scientists in applying online technologies, and particularly cyber-mapping tools, to address important social science questions,” she says, “The research allows us to examine the development and expansion of issue networks in a wholly new three dimensional space that means we can track the formation of alliances between groups over time and across countries.”
One cannot help but wonder whether all the money being spent on such endeavours, the cash ploughed into lobbying, and the cost of endlessly debating non-issues, could not be better spent by the nanoscientists themselves to investigate with even greater precision the properties and safety aspects of their discoveries. Surely, the money could assist the maturation of nano, rather than us having to see scientists fending off lobbyists and rebuilding labs, as opposed to GM crops, trashed by extremists.
Build a better mousetrap, they say, and the world will beat a path to your door. While, nano is not really anything but a question of scale, it really could be the better mousetrap we have been looking for, figuratively speaking. Advocates have to hope that those beating at their door are as keen to see the technology mature safely as they are.
Despite appearances, anyone hoping to get some kind of a buzz from a soft drink marketed as “Speed in a Can,” “Liquid Cocaine” and “Cocaine – Instant Rush”, will get nothing more than a potentially harmful spiking of their blood sugar concentration and a dash of the stimulant caffeine. The soda in a red can with an almost familiar logo, actually contains no illicit drugs and is supposedly marketed only as an energy drink and food supplement. The product has been sold since August 2006 in at least a dozen US states, but has come under attack from the FDA (Food and Drug Administration) and was this week pulled from supermarket shelves.
 As it is a holiday across the UK today, there is probably little need to warn Brits of the dangers of the sun’s rays, it’s usually so cold and wet, that the chances of frostbite and rust are much higher than sunburn. That said, summer is on its way and a paper in the latest edition of the Lancet medical journal warns that sunscreen and light cotton clothing are simply not enough to protect you from skin cancer caused by exposure to ultraviolet light from the sun.
As it is a holiday across the UK today, there is probably little need to warn Brits of the dangers of the sun’s rays, it’s usually so cold and wet, that the chances of frostbite and rust are much higher than sunburn. That said, summer is on its way and a paper in the latest edition of the Lancet medical journal warns that sunscreen and light cotton clothing are simply not enough to protect you from skin cancer caused by exposure to ultraviolet light from the sun. The Wolfram Demonstrations Project launched this week and represents what the developers describe as “a major new resource for research and education”. Well, that’s as maybe, but what is it? The project was first conceived by Stephen Wolfram creator of the Mathematica software that as its name suggests allows computers to produce visualisations of mathematical concepts.
The Wolfram Demonstrations Project launched this week and represents what the developers describe as “a major new resource for research and education”. Well, that’s as maybe, but what is it? The project was first conceived by Stephen Wolfram creator of the Mathematica software that as its name suggests allows computers to produce visualisations of mathematical concepts.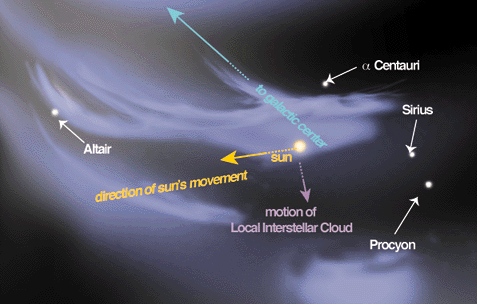 Sending astronauts up to our nearest star to reignite the Sun, the premise of sci-fi movie Sunshine, is truly the least of our problems when we are currently faced with global climate change, global terrorism, and global economic collapse. Nevertheless, astronomers are concerned about recent findings regarding the hot gas surrounding our star and its stellar neighbours. Put simply they cannot find them.
Sending astronauts up to our nearest star to reignite the Sun, the premise of sci-fi movie Sunshine, is truly the least of our problems when we are currently faced with global climate change, global terrorism, and global economic collapse. Nevertheless, astronomers are concerned about recent findings regarding the hot gas surrounding our star and its stellar neighbours. Put simply they cannot find them. Colourful language usually refers euphemistically to the kind of expletives and oaths you hear in a barrack room brawl. But, in the context of technology it could be the next big thing in colour printing.
Colourful language usually refers euphemistically to the kind of expletives and oaths you hear in a barrack room brawl. But, in the context of technology it could be the next big thing in colour printing. Does nanotechnology pose a threat to humanity on a par with the threat we face from genetically modified (GM) foods? That is the question asked by various lobbying groups from the verdant greens to the confused consumers. But, governments, academics and commercial bodies see the issue from an entirely different perspective and are asking, will the nanotechnology industry face the same outpouring of hostility as that caused by genetically modified (GM) foods? The kind of outpouring that stymies and often destroys research that could benefit us all.
Does nanotechnology pose a threat to humanity on a par with the threat we face from genetically modified (GM) foods? That is the question asked by various lobbying groups from the verdant greens to the confused consumers. But, governments, academics and commercial bodies see the issue from an entirely different perspective and are asking, will the nanotechnology industry face the same outpouring of hostility as that caused by genetically modified (GM) foods? The kind of outpouring that stymies and often destroys research that could benefit us all. A female friend of a friend started on hormone replacement therapy (to treat quite severe early postmenopausal symptoms, and on the advice of her GP to reduce the risk of osteoporosis). The symptoms have all but been relieved (although it’s difficult to separate out the effects of the HRT hormones themselves from the phytoestrogens she imbibes from soy milk and other related foods).
A female friend of a friend started on hormone replacement therapy (to treat quite severe early postmenopausal symptoms, and on the advice of her GP to reduce the risk of osteoporosis). The symptoms have all but been relieved (although it’s difficult to separate out the effects of the HRT hormones themselves from the phytoestrogens she imbibes from soy milk and other related foods).