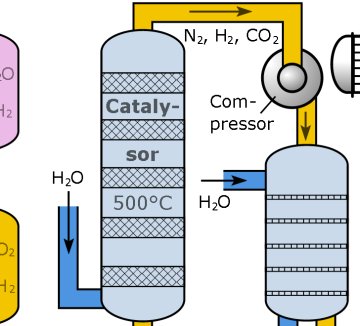
 It is a perennial on almost every chemistry course: the Haber-Bosch process for making ammonia. A potassium-doped iron catalyst is heated to several hundred degrees and high pressure nitrogen and hydrogen gas mixed over it to generate ammonia, which is then fed into fertiliser production. The H-B process very effectively traps and fixes nitrogen from the air, and is the industrial equivalent of nitrogen fixation by legumes and living other plants. The process, first demonstrated in 1909, has fed millions of people through the agricultural revolution of the twentieth century. You might say it has allowed our species to become so successful that it now numbers 7 billion…
It is a perennial on almost every chemistry course: the Haber-Bosch process for making ammonia. A potassium-doped iron catalyst is heated to several hundred degrees and high pressure nitrogen and hydrogen gas mixed over it to generate ammonia, which is then fed into fertiliser production. The H-B process very effectively traps and fixes nitrogen from the air, and is the industrial equivalent of nitrogen fixation by legumes and living other plants. The process, first demonstrated in 1909, has fed millions of people through the agricultural revolution of the twentieth century. You might say it has allowed our species to become so successful that it now numbers 7 billion…
However, that high temperature, even taking into account the industrial chemical engineer’s need for good quality “waste” heat for distillation towers and the like, is still wasteful. Catalysts that operate at a much lower temperature would be useful. It would also be useful if they could be dissolved rather than being solids, that would improve efficiency considerably given that the bottleneck in the H-B process is the release of nitrogen from the catalyst surface.
I just covered research from Patrick Holland and colleagues at the University of Rochester, New York, USA and the Max-Planck-Institut für Bioanorganische in Mülheim an der Ruhr, Germany, who have developed an iron compound that can promote the H-B process. You can read my full report on the research this week in Chemistry World.
Holland told me a bit more about his hopes for the research:
“I can envision two possibilities [in enhancing the energy efficiency of the H-B process],” he told me. “First, the structural information from our compounds could be used to design new solid surfaces for the H-B catalyst. In this way, we help surface scientists to formulate useful targets for the synthesis of solid catalysts. Secondly, if we could ultimately tune our solution complexes to make a catalyst that turns over many times in solution at room temperature, then it would reduce the high temperatures and pressures that are currently needed in the H-B process.”
I was curious as to what benefits such a catalyst might have over the standard catalysts that have been used and optimised over the last 100 years. “The high temperatures and pressures used in the H-B process place severe requirements on the factory,” Holland added, “after all, they use high-pressure hydrogen at hundreds of degrees Celsius! It is interesting to consider the reasons for these extreme conditions. The H-B process has a delta-G near zero, so the process is thermodynamically feasible but the rate of catalysis is slow. The reason it must be heated is to give an acceptable rate. Because the Haber-Bosch process is entropically unfavourable, the equilibrium becomes unfavourable at a high temperature unless the pressure is raised. So, the only reason for the high pressure is the high temperature that is needed for catalysis! A faster catalyst would work at lower temperatures, and require lower pressures too.”
There are several obstacles to surmount before a new low-temperature catalyst is ever available for ammonia production. “The current obstacles are that we need to make the final hydride product bind dinitrogen to ‘turn over’ [catalytically speaking] and secondly we need to increase the yields of the nitrogen and hydrogen reactions,” Holland told me. He adds that, “We have other related hydride complexes that can be converted into dinitrogen complexes, and so the situation is hopeful with respect to the first point. We are optimistic that by tuning the supporting ligands we can solve both of these problems and converge on a catalytic system.”
![]() Meghan M. Rodriguez, Eckhard Bill, William W. Brennessel, Patrick L. Holland (2011). N2 Reduction and Hydrogenation to Ammonia by a Molecular Iron-Potassium Complex Science, 334, 780-783 : 10.1126/science.1211906
Meghan M. Rodriguez, Eckhard Bill, William W. Brennessel, Patrick L. Holland (2011). N2 Reduction and Hydrogenation to Ammonia by a Molecular Iron-Potassium Complex Science, 334, 780-783 : 10.1126/science.1211906