Advanced photons in science
David Bradley is currently working with Argonne National Laboratory on a series of articles for the annual report of the ANL's Advanced Photon Source. Elemental Discoveries offers a preview of the advanced science featured in my report.

 Are films ferroelectric?
Are films ferroelectric?
Discipline for gold nanocrystals
Photosynthetic system
Dissecting the atom
Catalytic clues
SAXS and the water channel
Digging in the dirt
Folding Protein Sensors
X-ray movies - Repeated excitations reveal rapid geometric changes in real-time
A new x-ray technique that uses laser light to excite molecules and x-rays
to capture a snapshot of the molecules as they change shape can provide
chemists and biologists with detailed information about important catalytic,
photochemical and biological processes. The technique can provide
atom-by-atom detail about the short-lived chemical species that exist in the
short time span between reactant and product and so help researchers improve
industrially important reactions and better understand biochemistry.
Crystallography traditionally provides detailed snapshots of molecules only
in their lowest energy state, which researchers commonly refer to as the
"ground state". However, the ground state is not necessarily the most
interesting when it comes to understanding the behavior of different
molecules and how they interact with each other. Philip Coppens (pictured) of the
University of New York at Buffalo, realized that he might be able to probe
other energy states using x-ray techniques and so be able to watch geometric
changes as they take place at the molecular level. He and his colleagues
have pioneered a stroboscopic technique that uses laser light to repeatedly
excite the molecules in a sample and x-ray pulses on the ID-15 beamline to
capture an image of the short-lived species that exist in a transient
excited state between reactant and product.
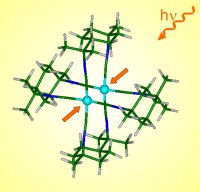 Coppens' recent experiment explores the reaction of a molecule containing
two atoms of the metal rhodium, and shows how these atoms react to exposure
to intense light from a laser source. The resulting excited (i.e. high
energy) species are highly reactive. The team used a burst of laser light
that lasts a billionth of a second and has an energy of just a few
hundred-thousandths of a joule. Immediately after excitation they expose the
molecules to a short pulse of x-rays from the ID-15 beamline. This allowed
them to probe the structure of the excited molecule several hundred thousand
times a second as it changes and drops back to its non-excited state ready
for the next burst of laser light. The method provides an instantaneous
snapshot of the molecule as it distorts in the high-energy laser beam.
Coppens' recent experiment explores the reaction of a molecule containing
two atoms of the metal rhodium, and shows how these atoms react to exposure
to intense light from a laser source. The resulting excited (i.e. high
energy) species are highly reactive. The team used a burst of laser light
that lasts a billionth of a second and has an energy of just a few
hundred-thousandths of a joule. Immediately after excitation they expose the
molecules to a short pulse of x-rays from the ID-15 beamline. This allowed
them to probe the structure of the excited molecule several hundred thousand
times a second as it changes and drops back to its non-excited state ready
for the next burst of laser light. The method provides an instantaneous
snapshot of the molecule as it distorts in the high-energy laser beam.
In an application of their new approach, Coppens' team performed a study on
an organometallic compound of rhodium in which the rhodium atoms are held
together by a "bridging" organic molecule. When this molecule is excited by
a burst of laser light, the rhodium metal atoms become linked by a direct
bond and are drawn much closer together than they are in the ground state of
the molecule. This, says Coppens, is the first time that scientists have
been able to observe such a light-induced molecular contraction directly
with detail revealed with atomic resolution.
Chemical and biological processes proceed through the formation of transient
chemical species that exist for only a few millionths of a second or less.
By probing the structure of such transient species researchers can gain
insights into the workings of all kinds of chemical and biochemical
reactions, from the catalytic conversion of raw materials into products to
the metabolic reactions that turn sugars into energy in our cells.
The energy stored for very brief times in such molecules can in turn be used
to initiate other chemical reactions. Related compounds, several of which
Coppens and his colleagues have also studied, are used to capture energy
from sunlight for the development of renewable energy resources.
Coppens explains that the further development of this technique to obtain
shorter time-resolution will allow even shorter-lived light-emitting species
to be studied in great detail and to allow researchers to monitor
light-induced reactions at the atomic level in real-time. The insights that
will become available from such studies will allow chemists to synthesize
new complexes with desirable properties, such as strong light emission and
light-to-energy conversion, that mimic biochemical processes such as
photosynthesis.
See Philip Coppens,1 Oksana Gerlits, 1 Ivan I. Vorontsov, 1 Andrey Yu.
Kovalevsky, 1 Yu-Sheng Chen, 2 Tim Graber, 3 Milan Gembicky, 1and Irina V.
Novozhilova,1 "A very large Rh-Rh bond shortening on excitation of the
[Rh2(1,8-diisocyano-p-menthane)4]2+ ion by time-resolved synchrotron X-ray
diffraction," Chem Commun, 2144-2145 (2004).
Author affiliation 1Department of Chemistry, SUNY at Buffalo, Buffalo, New
York, USA
2University of Toledo, Ohio 43606, USA 3University of Chicago, Illinois
60637, USA
Time-resolved experiments at the ID-15 beamline are supported through DOE
grant DE-FG02-02ER15372. Work at SUNY/Buffalo funded by the National Science
Foundation CHE0236317). The 15-ID beamline is funded through NSF CHE0087817.
Use of the Advanced Photon Source is supported by the US Department of
Energy, Office of Basic Energy Sciences, under Contract No. W-31-109-ENG-38.
Back to my Argonne National Laboratory Advanced Photon Source