UPDATE: If you have been prescribed this drug for another condition, do not stop taking it without consulting your GP. Your GP will have prescribed it for a good reason and will know your medical history and undertaken a risk-benefit assessment before signing your prescription. The majority of the side-effects are rare and it is generally safe to use for the approved conditions if heart problems and other underlying possible complications and contraindications have been ruled out for you.
In case you were ever stupid enough to follow Trump’s lead you would have already injected ultraviolets in your eyeballs by now to save you from Covid and maybe bathed in Domestos or sulfuric acid or both! Anyway, his latest bullshine claim is that he’s been taking the antimalarial drug hydroxychloroquine to keep Covid at bay.
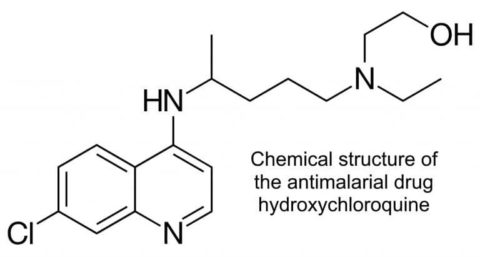
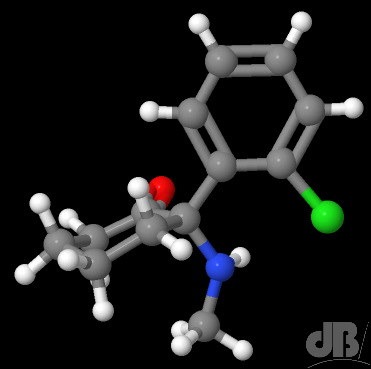
Well, for starters there is no evidence that this drug acts as a prophylactic against infection with SARS CoV-2 or indeed any pathogen other than the causative agent of otherwise drug-resistant malaria. It’s primary use is in treating lupus. There was some testing done weeks ago to see whether it might work against SARS-CoV-2, it almost certainly doesn’t, there will be actual antivirals to try and some being developed as we speak.
Either way, did anyone actually do a benefit-risk balance assessment for him or has he self-medicated on a whim? I strongly suspect that he is not taking it at all and that there is some hidden agenda. As with everything else he says bullshit or otherwise a political or financial incentive is often at the front of his frothing, festering mind.
The US Food and Drug Administration warns against taking this drug outside the clinical environment because it can cause serious and potentially lethal heart problems”. If you were thinking of taking inspiration from The Trunt listen to the FDA or maybe have a look at the huge list of post-approval adverse reactions and side-effects already reported for hydroxychloroquine:
Bone marrow failure, anemia, aplastic anemia, agranulocytosis, leukopenia, and thrombocytopenia, hemolysis in people with G-6-PD enzyme deficiency, Cardiomyopathy and fatal cardiac failure, ventricular arrhythmia, Vertigo, tinnitus, nystagmus, nerve deafness, deafness, eye irreversible retinopathy with retinal pigmentation changes (bull’s eye appearance), visual field defects, and visual disturbances, macular degeneration, corneal edema and opacity, nausea, vomiting, diarrhea, and abdominal pain, fatigue, liver failure, urticaria, angioedema, asthma, poor appetite, hypoglycemia, porphyria, weight loss, sensorimotor disorder, skeletal muscle myopathy or neuromyopathy leading to weakness and muscle wasting, failing tendon reflexes and abnormal nerve conduction, headache, dizziness, seizure, ataxia and dystonia, dyskinesia, and tremor, emotional problems, nervousness, irritability, nightmares, psychosis, suicidal thoughts, rash, pruritus, pigmentation disorders in skin and mucous membranes, alopecia, skin eruptions, toxic epidermal necrolysis, photosensitivity, psoriasis…
The complete, detailed list of ADRs and side-effects can be found here.