 The UK Times paper reported on Saturday that a leading cancer researcher Professor Lawrie Challis chairman of the government-funded mobile telecommunications health research programme believes it is time that a large-scale study into the long-term risks associated with cellphone use.
The UK Times paper reported on Saturday that a leading cancer researcher Professor Lawrie Challis chairman of the government-funded mobile telecommunications health research programme believes it is time that a large-scale study into the long-term risks associated with cellphone use.
Intriguingly, health and medicine writer Caroline Richmond pointed out that just such a study was actually published just three days prior to The Times article appearing.
The abstract for this paper by STUK, Radiation and Nuclear Safety Authority, Helsinki, Finland says:
“We conducted a population-based case-control study to investigate the relationship between mobile phone use and risk of glioma among 1,522 glioma patients and 3,301 controls. We found no evidence of increased risk of glioma related to regular mobile phone use (odds ratio, OR = 0.78, 95% confidence interval, CI: 0.68, 0.91).” The study encompasses digital and analog mobile phone use lasting ten years.
More than 200,000 volunteers and £3 million ($6m) of government and phone industry money will be needed to assess long-term risks of five years or so for cancer and Parkinson’s and Alzheimer’s diseases. Challis is currently negotiating for the necessary funding.
It is odd that this news story broke so close to the publication online in International Journal of Cancer. It also makes one wonder why there seems to be such a continued “hope” among certain segments of the media to find a correlation between mobile phone use and brain cancer. Surely, there isn’t an expectation that if such a correlation were ever demonstrated that the industry would cough up compensation to the literally millions upon millions of regular, long-term mobile phone users. Moreover, if such a demonstration were published might not a similar investigation raise concerns about other electromagnetic radiation sources again, such as powerlines, computer screens, microwave ovens and most recently wireless internet connections?
What do Sciencebase readers think? Would this be £3m well spent, or shouldn’t The Times simply publish a front page story about the STUK study, so similar to the one that Challis is after, that has already been carried out, peer reviewed and published.
 The UK Times paper reported on Saturday that a leading cancer researcher Professor Lawrie Challis chairman of the government-funded mobile telecommunications health research programme believes it is time that a large-scale study into the long-term risks associated with cellphone use.
The UK Times paper reported on Saturday that a leading cancer researcher Professor Lawrie Challis chairman of the government-funded mobile telecommunications health research programme believes it is time that a large-scale study into the long-term risks associated with cellphone use. Two back-to-back papers in the well-known chemistry journal Angewandte Chemie recently could have potentially serious consequences for the pharmaceutical industry, because they reveal what the authors claim are inherent ambiguities in the crystalline forms of aspirin.
Two back-to-back papers in the well-known chemistry journal Angewandte Chemie recently could have potentially serious consequences for the pharmaceutical industry, because they reveal what the authors claim are inherent ambiguities in the crystalline forms of aspirin.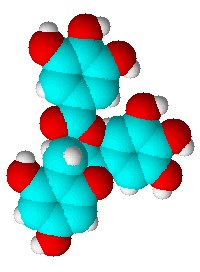 Researchers from Slovenia have used spectroscopy to home in on the active site of an essential bacterial enzyme, DNA gyrase. They say they now understand more clearly how a compound found in green tea, EGCG, which is a health-boosting antioxidant, works to kill bacteria.
Researchers from Slovenia have used spectroscopy to home in on the active site of an essential bacterial enzyme, DNA gyrase. They say they now understand more clearly how a compound found in green tea, EGCG, which is a health-boosting antioxidant, works to kill bacteria. A simple Q tip is all it takes to grab a microscopic sample from a work of art for laboratory testing, according to Canadian analytical chemists. They’ve used the approach to sample darkening pigments from an ancient map and from a piece of modern art as proof of principle.
A simple Q tip is all it takes to grab a microscopic sample from a work of art for laboratory testing, according to Canadian analytical chemists. They’ve used the approach to sample darkening pigments from an ancient map and from a piece of modern art as proof of principle.