 What’s the connection between Antarctic ice, old volcanic eruptions and global warming? US researchers think they know.
What’s the connection between Antarctic ice, old volcanic eruptions and global warming? US researchers think they know.
Volcanic activity can have serious consequences for climate change as particles and gases spewed out by volcanoes enter the upper atmosphere and change its chemical balance altering how Solar radiation is absorbed or reflected. Now, French and US researchers have devised a technique for determining how past volcanic eruptions could have affected this delicate chemical balance. Their findings could reduce significantly the uncertainty in current models of global climate change and so provide more accurate predictions of future global temperatures.
Joël Savarino of the National Center for Scientific Research (CNRS) and the University of Grenoble in France together with colleagues at the University of California San Diego have reported the pattern of sulphur isotopes of volcanic fallout from past eruptions. They also determined how far into the upper atmosphere the volcanic material reached, and what chemical reactions might have occurred there.
More…
 Light-emitting diodes almost ubiquitously provide the illumination in electronics and potentially will provide energy-efficient brightness in our homes. However, the LED material of choice, gallium nitride, and its method of processing and manufacture into working devices is relatively expensive. Now, US engineers have developed a novel semiconducting material based on zinc oxide that could be used in a new type of LED that is just as effective but could reduce costs for a wide range of applications.
Light-emitting diodes almost ubiquitously provide the illumination in electronics and potentially will provide energy-efficient brightness in our homes. However, the LED material of choice, gallium nitride, and its method of processing and manufacture into working devices is relatively expensive. Now, US engineers have developed a novel semiconducting material based on zinc oxide that could be used in a new type of LED that is just as effective but could reduce costs for a wide range of applications. Regular readers will recall my mention of the
Regular readers will recall my mention of the 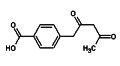 Spam comes in all shapes and forms, so I am always suspicious when two emails identical in content and with attachments arrive that purport to be from two different correspondents. However, two such messages arrived this morning one claiming to come from a Dr Suhasini Bhatnagar, the other from Aarif Khatri. Normally, I’d let my spam filter do its job and trash such messages, but my interest was piqued by the subject line, which read “help regarding synthesis”. Often spam arrives with two random words stuck together that are supposed to beat spam filters, but three is rare and even less frequent are subject lines that make logical sense and simultaneously are pertinent to my interests.
Spam comes in all shapes and forms, so I am always suspicious when two emails identical in content and with attachments arrive that purport to be from two different correspondents. However, two such messages arrived this morning one claiming to come from a Dr Suhasini Bhatnagar, the other from Aarif Khatri. Normally, I’d let my spam filter do its job and trash such messages, but my interest was piqued by the subject line, which read “help regarding synthesis”. Often spam arrives with two random words stuck together that are supposed to beat spam filters, but three is rare and even less frequent are subject lines that make logical sense and simultaneously are pertinent to my interests.